Electron spin resonance (ESR) detects unpaired electron spins and is highly sensitive to paramagnetic species, while nuclear magnetic resonance (NMR) focuses on the magnetic properties of atomic nuclei, providing detailed structural information about molecules. Discover how these powerful spectroscopic techniques differ and which one suits your research needs by reading the full article.
Table of Comparison
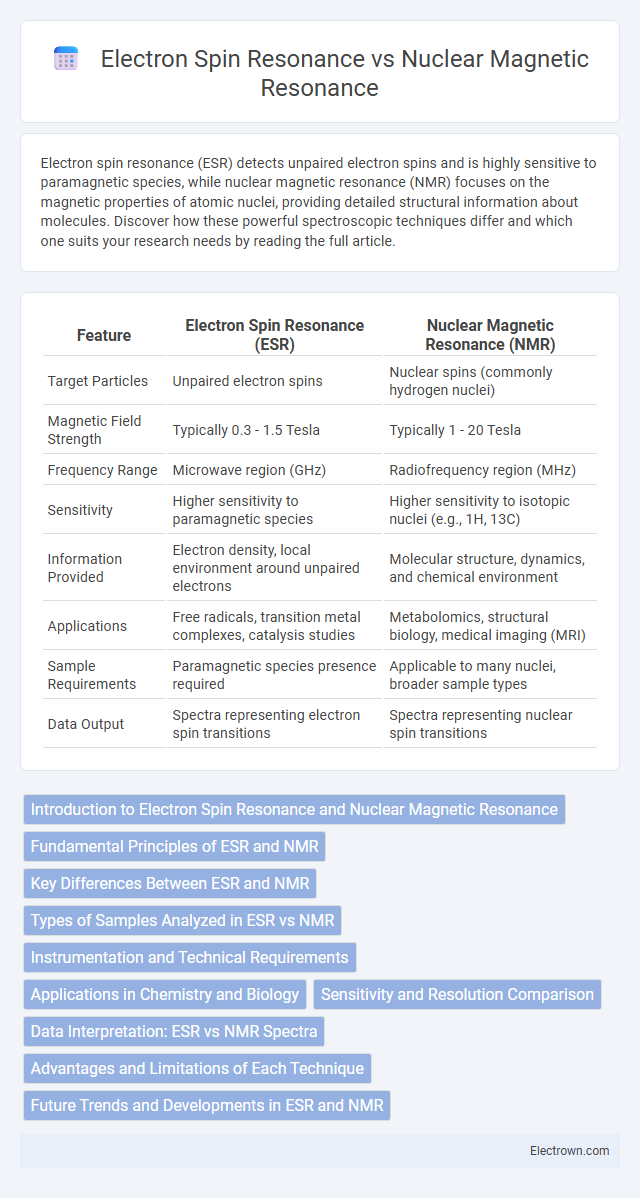
| Feature | Electron Spin Resonance (ESR) | Nuclear Magnetic Resonance (NMR) |
|---|---|---|
| Target Particles | Unpaired electron spins | Nuclear spins (commonly hydrogen nuclei) |
| Magnetic Field Strength | Typically 0.3 - 1.5 Tesla | Typically 1 - 20 Tesla |
| Frequency Range | Microwave region (GHz) | Radiofrequency region (MHz) |
| Sensitivity | Higher sensitivity to paramagnetic species | Higher sensitivity to isotopic nuclei (e.g., 1H, 13C) |
| Information Provided | Electron density, local environment around unpaired electrons | Molecular structure, dynamics, and chemical environment |
| Applications | Free radicals, transition metal complexes, catalysis studies | Metabolomics, structural biology, medical imaging (MRI) |
| Sample Requirements | Paramagnetic species presence required | Applicable to many nuclei, broader sample types |
| Data Output | Spectra representing electron spin transitions | Spectra representing nuclear spin transitions |
Introduction to Electron Spin Resonance and Nuclear Magnetic Resonance
Electron Spin Resonance (ESR) and Nuclear Magnetic Resonance (NMR) are powerful spectroscopic techniques used to study magnetic properties of materials at the atomic level. ESR detects unpaired electron spins, making it ideal for investigating free radicals and transition metal complexes, while NMR focuses on nuclear spins of isotopes like hydrogen-1, providing detailed information about molecular structure and dynamics. Your choice between ESR and NMR depends on whether you need insights into electron environments or nuclear configurations within the sample.
Fundamental Principles of ESR and NMR
Electron Spin Resonance (ESR) and Nuclear Magnetic Resonance (NMR) both rely on the magnetic properties of particles but differ in the type of spins they detect; ESR measures transitions of unpaired electron spins in a magnetic field, while NMR detects nuclear spin transitions of certain isotopes like hydrogen-1 or carbon-13. ESR requires a strong magnetic field and microwave radiation to induce electron spin transitions, exploiting the higher magnetic moment of electrons, whereas NMR uses radiofrequency radiation to excite nuclear spins subjected to an external magnetic field. The fundamental principles involve Zeeman splitting of spin states in both techniques, but ESR focuses on electron paramagnetic centers, providing detailed information on electronic environments, while NMR yields insights into molecular structure and dynamics through nuclear spin interactions.
Key Differences Between ESR and NMR
Electron Spin Resonance (ESR) detects unpaired electron spins typically found in paramagnetic species, whereas Nuclear Magnetic Resonance (NMR) focuses on the magnetic properties of atomic nuclei in molecules. ESR operates at higher frequencies due to the larger magnetic moment of electrons compared to nuclei, resulting in greater sensitivity to electronic environments. NMR provides detailed structural and compositional information about molecules, while ESR is primarily used to study free radicals and transition metal complexes.
Types of Samples Analyzed in ESR vs NMR
Electron Spin Resonance (ESR) primarily analyzes samples containing unpaired electrons, such as free radicals, transition metal complexes, and defects in solids, making it highly effective for studying paramagnetic species. Nuclear Magnetic Resonance (NMR), on the other hand, examines nuclei with non-zero spin in diamagnetic molecules, including organic compounds, biomolecules, and complex mixtures, providing detailed structural and dynamic information. Your choice between ESR and NMR depends on whether the sample exhibits paramagnetism or is predominantly diamagnetic.
Instrumentation and Technical Requirements
Electron Spin Resonance (ESR) requires a microwave source typically operating in the X-band (~9.5 GHz), a strong electromagnet capable of generating magnetic fields around 0.3-1.5 Tesla, and a resonant cavity designed for unpaired electron detection. Nuclear Magnetic Resonance (NMR) instrumentation relies on a strong superconducting magnet with fields commonly ranging from 1.5 to 23.5 Tesla, radiofrequency coils tuned to specific nuclear Larmor frequencies, and cryogenic probes for enhanced sensitivity. Both techniques necessitate precise magnetic field homogeneity and stability, but ESR generally demands higher microwave frequency sources and specialized cavities, while NMR emphasizes high-field superconducting magnets and advanced RF coil technology.
Applications in Chemistry and Biology
Electron spin resonance (ESR) provides detailed insights into free radicals and transition metal complexes, making it essential for studying reaction mechanisms and oxidative stress in chemistry and biology. Nuclear magnetic resonance (NMR) excels in elucidating molecular structures, dynamics, and interactions of biomolecules, enabling detailed analysis of proteins, nucleic acids, and metabolites. Your research benefits from ESR's sensitivity to unpaired electrons while NMR offers comprehensive structural information at the atomic level.
Sensitivity and Resolution Comparison
Electron spin resonance (ESR) exhibits higher sensitivity than nuclear magnetic resonance (NMR) due to the larger magnetic moment of unpaired electrons compared to nuclear spins, enabling detection of fewer spins at lower concentrations. However, NMR offers superior resolution in chemical structure analysis, as nuclear spins experience a wider range of chemical shift environments, providing detailed molecular information. ESR sensitivity thrives in paramagnetic species detection, while NMR resolution excels in elucidating complex organic and biological structures.
Data Interpretation: ESR vs NMR Spectra
Electron spin resonance (ESR) spectra exhibit broader linewidths and higher g-factor anisotropy due to interactions of unpaired electron spins with their environment, providing detailed information about paramagnetic centers. Nuclear magnetic resonance (NMR) spectra feature narrower peaks with chemical shift dispersion reflecting the electronic environment of nuclear spins, allowing precise structural elucidation of diamagnetic molecules. Data interpretation in ESR requires analyzing hyperfine splitting patterns influenced by electron-nuclear interactions, while NMR data focus on coupling constants and relaxation times to deduce molecular dynamics and connectivity.
Advantages and Limitations of Each Technique
Electron spin resonance (ESR) offers high sensitivity in detecting unpaired electrons, making it ideal for studying free radicals and transition metal ions, but it is limited by the rarity of paramagnetic species in biological samples. Nuclear magnetic resonance (NMR) provides detailed structural information on molecules in a non-destructive manner and is widely applicable to both liquids and solids, though it requires larger sample quantities and has lower sensitivity compared to ESR. Your choice between ESR and NMR depends on whether the focus is on electron-based paramagnetic centers or on comprehensive molecular structure and dynamics.
Future Trends and Developments in ESR and NMR
Future trends in Electron Spin Resonance (ESR) and Nuclear Magnetic Resonance (NMR) focus on enhancing sensitivity and resolution through advanced quantum sensors and hyperpolarization techniques. Integration of machine learning algorithms is accelerating data analysis and structural interpretation, enabling real-time applications in medical diagnostics and material science. Emerging developments also emphasize miniaturized, portable ESR and NMR devices for in-field chemical analysis and non-invasive biological monitoring.
electron spin resonance vs nuclear magnetic resonance Infographic
 electrown.com
electrown.com