Spin-orbit coupling arises from the interaction between an electron's spin and its orbital motion around the nucleus, influencing energy level splitting and magnetic properties in atoms and molecules. Understanding the differences between spin-orbit and spin-spin coupling is crucial for interpreting spectroscopic data and molecular behavior, so explore the rest of this article to deepen your knowledge of these quantum phenomena.
Table of Comparison
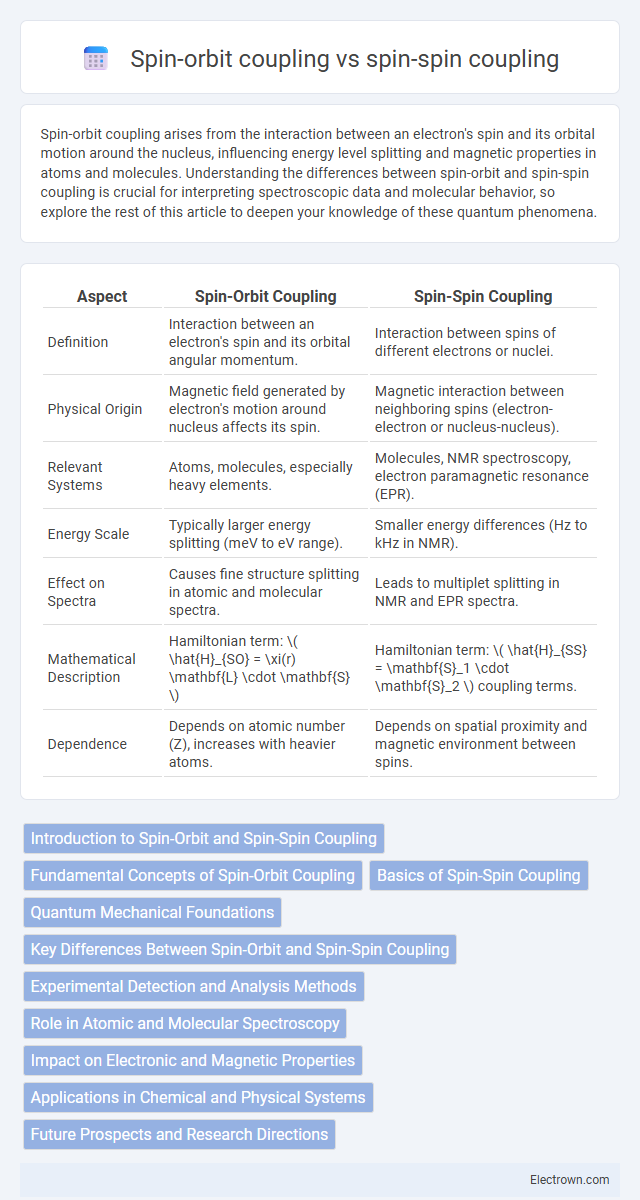
| Aspect | Spin-Orbit Coupling | Spin-Spin Coupling |
|---|---|---|
| Definition | Interaction between an electron's spin and its orbital angular momentum. | Interaction between spins of different electrons or nuclei. |
| Physical Origin | Magnetic field generated by electron's motion around nucleus affects its spin. | Magnetic interaction between neighboring spins (electron-electron or nucleus-nucleus). |
| Relevant Systems | Atoms, molecules, especially heavy elements. | Molecules, NMR spectroscopy, electron paramagnetic resonance (EPR). |
| Energy Scale | Typically larger energy splitting (meV to eV range). | Smaller energy differences (Hz to kHz in NMR). |
| Effect on Spectra | Causes fine structure splitting in atomic and molecular spectra. | Leads to multiplet splitting in NMR and EPR spectra. |
| Mathematical Description | Hamiltonian term: \( \hat{H}_{SO} = \xi(r) \mathbf{L} \cdot \mathbf{S} \) | Hamiltonian term: \( \hat{H}_{SS} = \mathbf{S}_1 \cdot \mathbf{S}_2 \) coupling terms. |
| Dependence | Depends on atomic number (Z), increases with heavier atoms. | Depends on spatial proximity and magnetic environment between spins. |
Introduction to Spin-Orbit and Spin-Spin Coupling
Spin-orbit coupling arises from the interaction between an electron's spin and its orbital angular momentum, significantly influencing atomic and molecular spectra by causing energy level splitting. Spin-spin coupling, on the other hand, results from magnetic interactions between spins of different electrons or nuclei, crucial in electron paramagnetic resonance (EPR) and nuclear magnetic resonance (NMR) spectroscopy for revealing structural information. These coupling mechanisms play fundamental roles in quantum mechanics and spectroscopy by affecting magnetic properties and energy transitions of particles.
Fundamental Concepts of Spin-Orbit Coupling
Spin-orbit coupling arises from the interaction between an electron's spin and its orbital motion around the nucleus, influencing energy levels and spectral lines in atoms. This quantum mechanical effect is crucial for understanding fine structure in atomic spectra and impacts magnetic and electronic properties in materials. Your study of spin interactions will benefit from distinguishing spin-orbit coupling, which depends on the electron's motion and angular momentum, from spin-spin coupling involving magnetic interactions between separate electron spins.
Basics of Spin-Spin Coupling
Spin-spin coupling, also known as scalar coupling, occurs between nuclear spins through chemical bonds and provides critical information about molecular structure in NMR spectroscopy. Unlike spin-orbit coupling, which involves the interaction between an electron's spin and its orbital motion, spin-spin coupling reflects the magnetic interactions between neighboring nuclear spins, resulting in multiplet splitting patterns. Your analysis of spin-spin coupling helps determine connectivity and spatial relationships between atoms in a molecule.
Quantum Mechanical Foundations
Spin-orbit coupling arises from the interaction between an electron's spin and its orbital angular momentum, fundamentally linked to relativistic corrections in quantum mechanics. Spin-spin coupling, also known as electron spin-spin interaction, occurs due to magnetic dipole-dipole interactions between unpaired electron spins in multi-electron systems, governed by the Heisenberg exchange Hamiltonian. Both phenomena are critical in determining fine and hyperfine spectral structures in atomic, molecular, and solid-state physics, reflecting different quantum mechanical operators and symmetries.
Key Differences Between Spin-Orbit and Spin-Spin Coupling
Spin-orbit coupling involves the interaction between an electron's spin and its orbital motion around the nucleus, resulting in energy level splitting that influences fine spectral structures in atoms. Spin-spin coupling occurs due to interactions between the spins of neighboring nuclei or electrons, significantly affecting the splitting patterns observed in nuclear magnetic resonance (NMR) and electron spin resonance (ESR) spectroscopy. Understanding these distinct mechanisms helps you interpret spectral data accurately, as spin-orbit coupling primarily affects electronic energy levels while spin-spin coupling impacts nuclear or electron spin interactions.
Experimental Detection and Analysis Methods
Spin-orbit coupling is commonly detected through techniques such as electron spin resonance (ESR) and angle-resolved photoemission spectroscopy (ARPES), which reveal interactions between electron spin and orbital angular momentum by analyzing splitting patterns in energy spectra. Spin-spin coupling is primarily studied using nuclear magnetic resonance (NMR) and electron paramagnetic resonance (EPR) spectroscopy to measure hyperfine splitting resulting from interactions between neighboring spins. Your choice of analysis method depends on the specific coupling type and the resolution needed to characterize spin interactions within the material or molecule.
Role in Atomic and Molecular Spectroscopy
Spin-orbit coupling significantly influences atomic and molecular spectroscopy by causing fine structure splitting of spectral lines, resulting from the interaction between an electron's spin and its orbital angular momentum. Spin-spin coupling, on the other hand, primarily affects molecular spectroscopy through hyperfine structure splitting, emerging from interactions between the spins of different electrons or nuclei. These couplings provide critical insights into electronic configurations, molecular geometry, and magnetic properties in spectroscopic analysis.
Impact on Electronic and Magnetic Properties
Spin-orbit coupling significantly influences electronic band structures and magnetic anisotropy by linking electron spin with its orbital motion, thereby affecting phenomena like spin Hall effect and magnetic anisotropy energy. Spin-spin coupling primarily governs the interaction between electron spins, impacting magnetic resonance behaviors and spin relaxation times in materials. Understanding these interactions helps optimize Your designs in spintronic devices and magnetic materials by tailoring electronic and magnetic properties for desired performance.
Applications in Chemical and Physical Systems
Spin-orbit coupling plays a crucial role in spectroscopy and quantum chemistry, influencing electronic transitions and enabling fine structure splitting in atomic and molecular spectra. Spin-spin coupling is essential in nuclear magnetic resonance (NMR) spectroscopy, providing detailed information on molecular structure, conformation, and dynamics through scalar coupling constants. Both interactions are fundamental in material science for understanding magnetic properties, electron spin behaviors, and designing spintronic devices.
Future Prospects and Research Directions
Future prospects in spin-orbit coupling research focus on quantum computing advancements by exploiting spintronic devices with enhanced coherence times. Spin-spin coupling studies aim to improve magnetic resonance imaging techniques and quantum information processing through precise manipulation of spin interactions. Exploring hybrid systems combining both couplings could unlock new pathways for ultra-sensitive sensors and scalable quantum networks tailored to your technological needs.
spin-orbit coupling vs spin-spin coupling Infographic
 electrown.com
electrown.com