Nitrogen-vacancy centers and quantum dots serve as critical components in quantum computing and sensing, with nitrogen-vacancy centers offering stable spin states at room temperature while quantum dots excel in tunable electronic properties and light emission. Explore the article to understand how these unique quantum materials compare and which one best fits your technology needs.
Table of Comparison
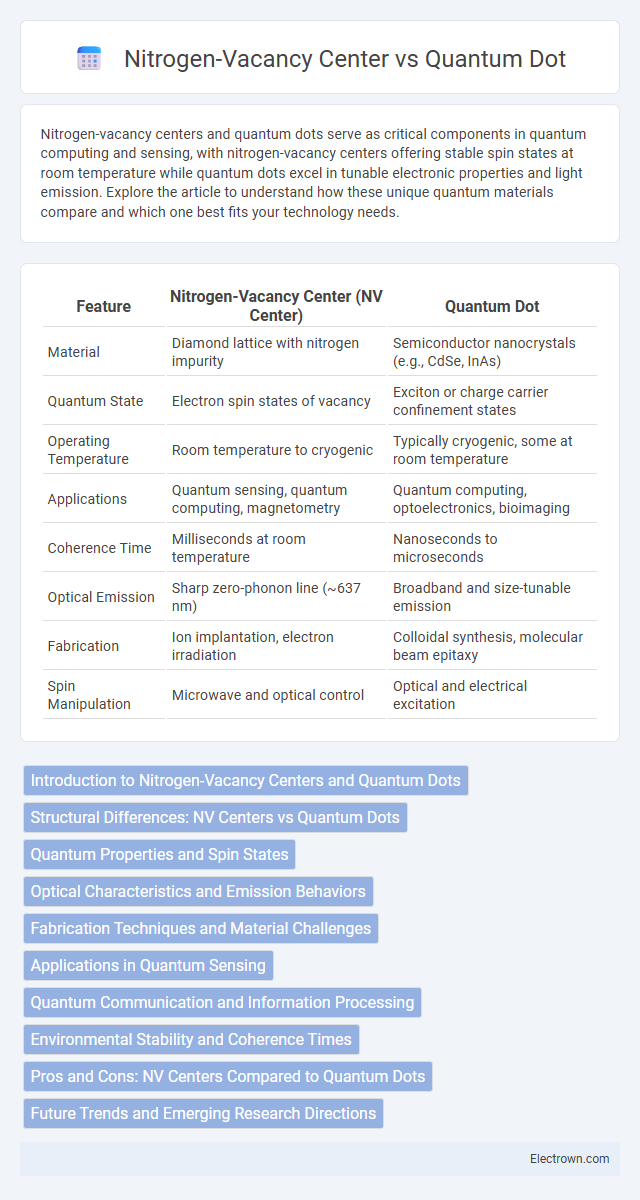
| Feature | Nitrogen-Vacancy Center (NV Center) | Quantum Dot |
|---|---|---|
| Material | Diamond lattice with nitrogen impurity | Semiconductor nanocrystals (e.g., CdSe, InAs) |
| Quantum State | Electron spin states of vacancy | Exciton or charge carrier confinement states |
| Operating Temperature | Room temperature to cryogenic | Typically cryogenic, some at room temperature |
| Applications | Quantum sensing, quantum computing, magnetometry | Quantum computing, optoelectronics, bioimaging |
| Coherence Time | Milliseconds at room temperature | Nanoseconds to microseconds |
| Optical Emission | Sharp zero-phonon line (~637 nm) | Broadband and size-tunable emission |
| Fabrication | Ion implantation, electron irradiation | Colloidal synthesis, molecular beam epitaxy |
| Spin Manipulation | Microwave and optical control | Optical and electrical excitation |
Introduction to Nitrogen-Vacancy Centers and Quantum Dots
Nitrogen-vacancy (NV) centers in diamond are atomic-scale defects where a nitrogen atom replaces a carbon atom adjacent to a vacancy, exhibiting remarkable quantum coherence properties ideal for quantum sensing and information processing. Quantum dots are semiconductor nanocrystals with size-tunable electronic and optical properties, enabling applications in quantum computing, photovoltaics, and bio-imaging. Both NV centers and quantum dots serve as solid-state qubits but differ fundamentally in structure, material composition, and operational mechanisms.
Structural Differences: NV Centers vs Quantum Dots
Nitrogen-vacancy (NV) centers are point defects in diamond consisting of a nitrogen atom adjacent to a vacancy in the carbon lattice, creating a stable and highly coherent quantum system. Quantum dots, in contrast, are semiconductor nanocrystals whose electronic properties are governed by quantum confinement effects within a finite particle-sized structure. Understanding these structural differences is essential for optimizing Your choice of quantum technology applications such as sensing, imaging, or quantum computing.
Quantum Properties and Spin States
Nitrogen-vacancy centers in diamond exhibit stable spin states that can be optically initialized and read out at room temperature, making them ideal for quantum sensing and information processing. Quantum dots offer tunable electronic and spin properties through size and material composition, enabling controlled quantum state manipulation with strong coupling to photons. Both systems enable coherent spin control, but nitrogen-vacancy centers provide longer spin coherence times, while quantum dots excel in integrability with semiconductor technology.
Optical Characteristics and Emission Behaviors
Nitrogen-vacancy (NV) centers in diamond exhibit stable photoluminescence with narrow zero-phonon lines around 637 nm, making them highly suitable for quantum sensing and single-photon emission at room temperature. Quantum dots display size-tunable emission spectra ranging from visible to near-infrared wavelengths, characterized by broad photoluminescence peaks and high quantum yields, which are advantageous for bioimaging and optoelectronics. Understanding these distinct optical characteristics allows you to select the appropriate nanomaterial for specific applications requiring precise emission behaviors and stability.
Fabrication Techniques and Material Challenges
Nitrogen-vacancy (NV) centers in diamond are typically fabricated through ion implantation followed by high-temperature annealing, requiring precise control over defect positioning and minimal lattice damage to maintain coherence properties. Quantum dots, often synthesized via colloidal methods or epitaxial growth like molecular beam epitaxy (MBE), face challenges related to size uniformity and surface defect passivation to ensure consistent optical and electronic behavior. Your choice between NV centers and quantum dots depends heavily on the material system's compatibility with intended quantum applications and the ability to optimize fabrication parameters for scalability and device integration.
Applications in Quantum Sensing
Nitrogen-vacancy (NV) centers in diamond offer exceptional sensitivity for magnetic and electric field detection, making them ideal for high-precision quantum sensing applications such as nanoscale magnetometry and temperature measurement. Quantum dots, while also used in quantum sensing, excel in optical properties and fluorescence stability, enabling applications in biological imaging and single-photon detection. Your choice between NV centers and quantum dots depends on whether you require atomic-scale sensing with high spatial resolution or versatile optical readout capabilities in your quantum sensing tasks.
Quantum Communication and Information Processing
Nitrogen-vacancy (NV) centers in diamond offer robust spin coherence and optical addressability at room temperature, making them ideal for quantum communication and information processing applications. Quantum dots provide tunable electronic and optical properties with fast emission rates, enabling scalable quantum networks and on-demand single-photon sources. While NV centers excel in long-term quantum memory and spin-based quantum sensors, quantum dots are preferred for integration into photonic circuits requiring high photon indistinguishability.
Environmental Stability and Coherence Times
Nitrogen-vacancy (NV) centers in diamond exhibit exceptional environmental stability and long coherence times, often exceeding milliseconds at room temperature, making them ideal for quantum sensing and information applications. Quantum dots generally suffer from shorter coherence times due to environmental fluctuations and charge noise but can be engineered for improved stability through surface passivation and embedding in optimized matrices. Your choice between NV centers and quantum dots depends on the required coherence duration and stability under ambient conditions for your specific quantum technology.
Pros and Cons: NV Centers Compared to Quantum Dots
Nitrogen-vacancy (NV) centers in diamond offer exceptional spin coherence times and operate effectively at room temperature, providing advantages over quantum dots that often require cryogenic conditions. NV centers exhibit stable fluorescence and high sensitivity for quantum sensing, but their fabrication and integration into scalable devices can be more challenging compared to the tunability and size variability of quantum dots. Quantum dots excel in photonic applications due to their wavelength tunability and ease of integration into semiconductor devices, though they generally suffer from shorter coherence times and susceptibility to environmental noise relative to NV centers.
Future Trends and Emerging Research Directions
Emerging research in nitrogen-vacancy (NV) centers emphasizes enhanced quantum sensing capabilities and scalable quantum networks leveraging diamond-based platforms. Quantum dots are advancing toward tunable optoelectronic devices and integrated photonic circuits with improved coherence times and emission stability. Future trends highlight hybrid systems combining NV centers and quantum dots to exploit complementary quantum properties for next-generation quantum computing and communication technologies.
nitrogen-vacancy center vs quantum dot Infographic
 electrown.com
electrown.com