Spin-orbit interaction arises from the coupling between an electron's spin and its orbital motion around the nucleus, causing energy level splitting dependent on the electron's angular momentum. The Zeeman effect, on the other hand, refers to the splitting of energy levels under the influence of an external magnetic field, affecting the magnetic moments of electrons; discover how these phenomena differ and impact your understanding of atomic and quantum physics in the rest of this article.
Table of Comparison
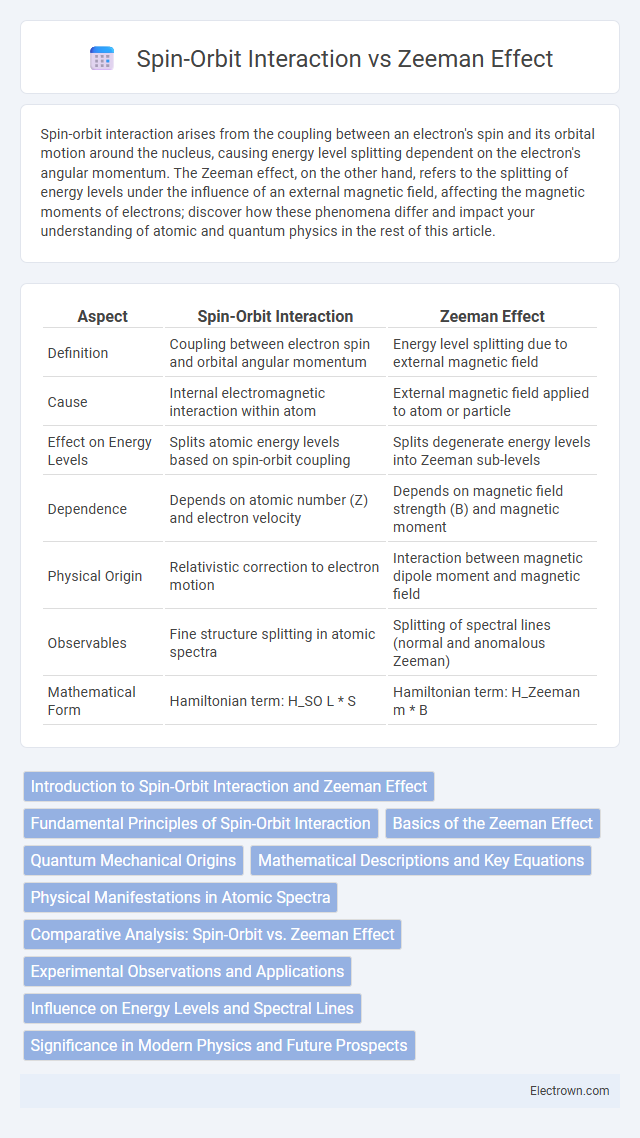
| Aspect | Spin-Orbit Interaction | Zeeman Effect |
|---|---|---|
| Definition | Coupling between electron spin and orbital angular momentum | Energy level splitting due to external magnetic field |
| Cause | Internal electromagnetic interaction within atom | External magnetic field applied to atom or particle |
| Effect on Energy Levels | Splits atomic energy levels based on spin-orbit coupling | Splits degenerate energy levels into Zeeman sub-levels |
| Dependence | Depends on atomic number (Z) and electron velocity | Depends on magnetic field strength (B) and magnetic moment |
| Physical Origin | Relativistic correction to electron motion | Interaction between magnetic dipole moment and magnetic field |
| Observables | Fine structure splitting in atomic spectra | Splitting of spectral lines (normal and anomalous Zeeman) |
| Mathematical Form | Hamiltonian term: H_SO L * S | Hamiltonian term: H_Zeeman m * B |
Introduction to Spin-Orbit Interaction and Zeeman Effect
Spin-orbit interaction arises from the coupling between an electron's spin and its orbital motion around the nucleus, influencing atomic energy levels and spectral lines. The Zeeman effect occurs when external magnetic fields split these energy levels further, causing shifts in spectral line positions based on magnetic quantum numbers. Understanding both phenomena is crucial for interpreting fine structure in atomic spectra and magnetic resonance measurements, giving you insights into electron behavior under varying magnetic conditions.
Fundamental Principles of Spin-Orbit Interaction
Spin-orbit interaction arises from the coupling between an electron's spin and its orbital motion around the nucleus, resulting in energy level splitting that depends on the relative orientation of spin and orbital angular momentum. This intrinsic quantum mechanical effect is fundamental to understanding fine structure in atomic spectra and crucial for phenomena such as spintronics and topological insulators. Your ability to manipulate spin-orbit coupling can significantly influence electron spin dynamics and magnetic properties in advanced materials.
Basics of the Zeeman Effect
The Zeeman effect describes the splitting of atomic energy levels in the presence of an external magnetic field due to the interaction between the magnetic moments of electrons and the field. It results in distinct spectral line patterns revealing information about the magnetic properties and electronic structure of atoms. Understanding the Zeeman effect is essential for interpreting magnetic resonance experiments and your research involving spin-related phenomena.
Quantum Mechanical Origins
Spin-orbit interaction arises from the coupling between an electron's spin and its orbital angular momentum due to relativistic corrections in quantum mechanics, where the electron experiences an effective magnetic field in its rest frame caused by its motion around the nucleus. The Zeeman effect originates from the interaction between an external magnetic field and the magnetic moment of electrons, resulting in energy level splitting proportional to the magnetic field strength and the electron's magnetic quantum number. Both effects modify atomic energy levels, but spin-orbit coupling is an intrinsic quantum property dependent on the electron's intrinsic spin and orbital angular momentum coupling, while the Zeeman effect is extrinsic, directly linked to the external magnetic environment.
Mathematical Descriptions and Key Equations
The spin-orbit interaction is mathematically described by the Hamiltonian \( H_{SO} = \xi(r) \mathbf{L} \cdot \mathbf{S} \), where \( \xi(r) \) represents the spin-orbit coupling strength dependent on the radial coordinate, \( \mathbf{L} \) is the orbital angular momentum operator, and \( \mathbf{S} \) is the spin angular momentum operator. The Zeeman effect is characterized by the Hamiltonian \( H_Z = -\boldsymbol{\mu} \cdot \mathbf{B} = g \mu_B \mathbf{S} \cdot \mathbf{B} \), where \( \boldsymbol{\mu} \) is the magnetic moment, \( g \) is the Lande g-factor, \( \mu_B \) is the Bohr magneton, and \( \mathbf{B} \) is the external magnetic field. Both interactions modify energy levels, with the spin-orbit coupling mixing spin and orbital characteristics, while the Zeeman effect splits energy levels based on spin orientation in a magnetic field.
Physical Manifestations in Atomic Spectra
Spin-orbit interaction causes fine structure splitting in atomic spectra by coupling an electron's spin with its orbital angular momentum, leading to energy level shifts within the same principal quantum number. The Zeeman effect produces spectral line splitting in the presence of an external magnetic field, altering energy levels based on the magnetic quantum number and spin orientation. These phenomena result in distinct multiplet patterns: spin-orbit interaction splits spectral lines intrinsically, while the Zeeman effect introduces splitting extrinsically depending on the magnetic field strength.
Comparative Analysis: Spin-Orbit vs. Zeeman Effect
Spin-orbit interaction arises from the coupling between an electron's spin and its orbital motion within an atom, causing energy level splitting dependent on the electron's angular momentum and nuclear charge. In contrast, the Zeeman effect results from an external magnetic field interacting with the electron's magnetic moment, leading to energy level shifts proportional to the magnetic field strength and electron spin orientation. Your understanding of atomic spectral lines can be enhanced by comparing how spin-orbit coupling is intrinsic and dependent on atomic structure, whereas the Zeeman effect is extrinsic and controlled by applied magnetic fields.
Experimental Observations and Applications
Experimental observations reveal that spin-orbit interaction leads to fine structure splitting in atomic spectra, observable through precise spectroscopic measurements, while the Zeeman effect manifests as the splitting of spectral lines in the presence of an external magnetic field. Spin-orbit coupling is crucial in spintronic devices for manipulating electron spin states without magnetic fields, enhancing data storage technologies. Zeeman effect applications include magnetic resonance imaging (MRI) and precision magnetometry, enabling accurate determination of magnetic field strengths in various materials.
Influence on Energy Levels and Spectral Lines
Spin-orbit interaction causes splitting of atomic energy levels by coupling an electron's spin with its orbital angular momentum, leading to fine structure in spectral lines. The Zeeman effect further splits these energy levels when an external magnetic field is applied, causing spectral lines to split into multiple components with characteristic polarization. Your spectral analysis reveals these distinct patterns, which are crucial for understanding atomic and molecular behavior in magnetic environments.
Significance in Modern Physics and Future Prospects
Spin-orbit interaction and the Zeeman effect play crucial roles in modern physics, shaping the understanding of electron behavior in atomic and condensed matter systems. You benefit from these phenomena in spintronics, quantum computing, and advanced spectroscopy, where controlling spin dynamics enables breakthroughs in information processing and material characterization. Future prospects include leveraging spin-orbit coupling and Zeeman splitting for topological quantum materials and ultra-sensitive magnetic sensors, promising transformative advancements across technology and fundamental science.
spin-orbit interaction vs Zeeman effect Infographic
 electrown.com
electrown.com