Silver ink offers superior electrical conductivity and stability in printed electrodes compared to carbon ink, which is valued for its cost-effectiveness and chemical resistance. Explore the full article to discover how these materials impact the performance and application of your printed electronics.
Table of Comparison
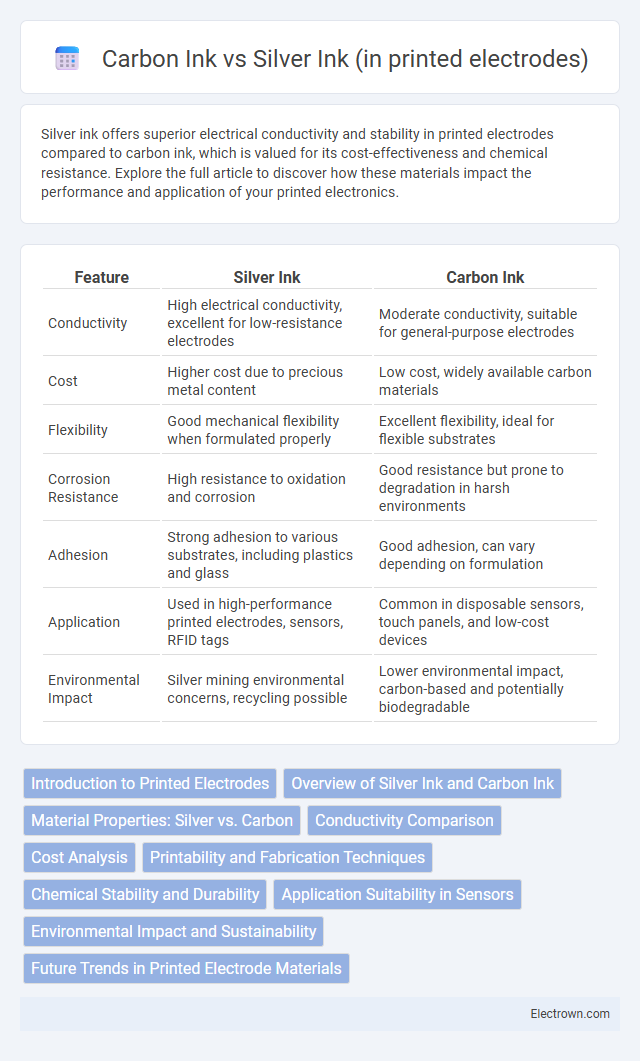
| Feature | Silver Ink | Carbon Ink |
|---|---|---|
| Conductivity | High electrical conductivity, excellent for low-resistance electrodes | Moderate conductivity, suitable for general-purpose electrodes |
| Cost | Higher cost due to precious metal content | Low cost, widely available carbon materials |
| Flexibility | Good mechanical flexibility when formulated properly | Excellent flexibility, ideal for flexible substrates |
| Corrosion Resistance | High resistance to oxidation and corrosion | Good resistance but prone to degradation in harsh environments |
| Adhesion | Strong adhesion to various substrates, including plastics and glass | Good adhesion, can vary depending on formulation |
| Application | Used in high-performance printed electrodes, sensors, RFID tags | Common in disposable sensors, touch panels, and low-cost devices |
| Environmental Impact | Silver mining environmental concerns, recycling possible | Lower environmental impact, carbon-based and potentially biodegradable |
Introduction to Printed Electrodes
Printed electrodes utilize conductive inks to achieve precise electrical pathways on flexible substrates, with silver ink and carbon ink being the most common materials. Silver ink offers superior conductivity and excellent signal stability, making it ideal for high-performance applications, while carbon ink provides cost-effective, chemically stable, and corrosion-resistant properties suitable for disposable sensors. Choosing between silver and carbon inks depends on factors such as conductivity requirements, biocompatibility, and device longevity in printed electrode fabrication.
Overview of Silver Ink and Carbon Ink
Silver ink, known for its excellent electrical conductivity and flexibility, is widely used in printed electrodes for applications requiring high-performance signal transmission. Carbon ink offers a cost-effective and chemically stable alternative with good conductivity, making it suitable for disposable or large-area printed electronics. Your choice between silver and carbon ink depends on balancing conductivity, durability, and budget requirements.
Material Properties: Silver vs. Carbon
Silver ink exhibits superior electrical conductivity, making it ideal for printed electrodes requiring low resistance and stable signal transmission. Carbon ink offers excellent chemical stability and flexibility, providing durability in harsh environments and mechanical stress. The choice between silver and carbon inks depends on the trade-off between conductivity and environmental resilience in printed electrode applications.
Conductivity Comparison
Silver ink exhibits significantly higher electrical conductivity compared to carbon ink, making it the preferred choice for printed electrodes requiring rapid electron transfer and low resistance. The conductivity of silver ink typically ranges around 10^6 S/m, whereas carbon ink's conductivity is substantially lower, often in the range of 10^3 to 10^4 S/m, impacting the overall sensor performance. This difference directly influences the sensitivity and response time of printed electrochemical devices.
Cost Analysis
Silver ink typically costs significantly more than carbon ink due to the high price of silver, impacting the overall budget for printed electrode production. Carbon ink offers a cost-effective alternative with lower material expenses, making it ideal for large-scale or disposable sensor applications. Your choice between these inks should balance performance requirements and cost-efficiency for the intended use.
Printability and Fabrication Techniques
Silver ink offers superior printability due to its high conductivity and stable particle dispersion, enabling fine pattern resolution in screen printing and inkjet techniques commonly used for printed electrodes. Carbon ink, while less conductive, excels in cost-effectiveness and environmental stability, making it suitable for extrusion and stencil printing methods where flexibility and large-area coverage are required. Both inks require precise control over viscosity and drying conditions to optimize electrode performance and ensure consistent layer formation during fabrication.
Chemical Stability and Durability
Silver ink exhibits superior chemical stability in printed electrodes due to its resistance to oxidation and corrosion, maintaining consistent conductivity over extended periods. Carbon ink, while cost-effective and flexible, tends to degrade faster under harsh environmental conditions, leading to reduced durability and performance. The inherent chemical inertness of silver ensures longer electrode lifespan in applications requiring reliable electrical conductivity.
Application Suitability in Sensors
Silver ink offers excellent electrical conductivity and biocompatibility, making it highly suitable for flexible and wearable sensors requiring precise signal transmission. Carbon ink, known for its chemical stability and cost-effectiveness, is ideal for eco-friendly or disposable sensors where durability and lower production expenses are prioritized. Your choice between silver and carbon inks depends on sensor application needs such as sensitivity, longevity, and environmental impact.
Environmental Impact and Sustainability
Silver ink, commonly used in printed electrodes, poses significant environmental concerns due to its resource-intensive mining process and limited recyclability, leading to metal waste and ecological disruption. Carbon ink offers a more sustainable alternative, derived from abundant and non-toxic materials like graphite or carbon black, reducing environmental impact and supporting circular economy practices. Choosing carbon ink for your printed electrodes promotes eco-friendly manufacturing and aligns with sustainability goals in electronics production.
Future Trends in Printed Electrode Materials
Future trends in printed electrode materials highlight a shift towards hybrid formulations combining silver ink's high conductivity and carbon ink's cost-efficiency and flexibility. Research advances emphasize improving the durability and environmental sustainability of silver-based inks while enhancing the electrochemical stability and conductivity of carbon inks through nanomaterial integration. Emerging applications in flexible electronics, wearable sensors, and energy storage devices drive the development of composite inks that leverage the distinct advantages of both silver and carbon materials.
Silver Ink vs Carbon Ink (in printed electrodes) Infographic
 electrown.com
electrown.com