Electrochemical sensors detect chemical changes through electrical signals, offering high sensitivity for gases and ions, while photonic sensors utilize light interactions to measure physical or chemical properties with precise optical detection. Discover how these two sensor technologies compare and which one fits your specific application by reading the full article.
Table of Comparison
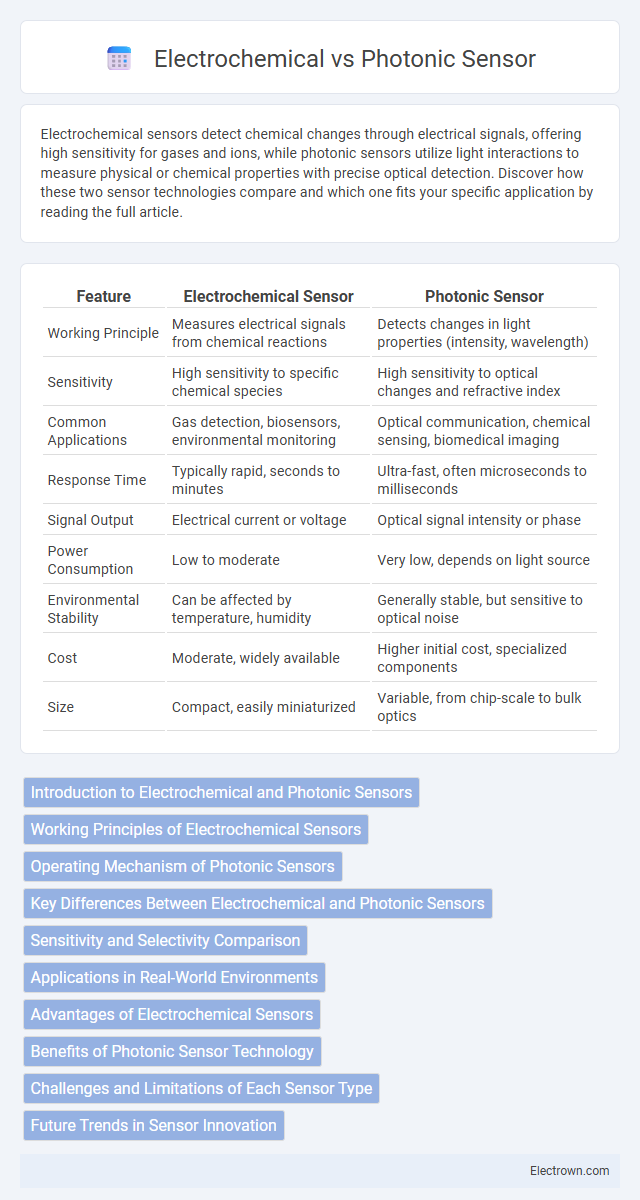
| Feature | Electrochemical Sensor | Photonic Sensor |
|---|---|---|
| Working Principle | Measures electrical signals from chemical reactions | Detects changes in light properties (intensity, wavelength) |
| Sensitivity | High sensitivity to specific chemical species | High sensitivity to optical changes and refractive index |
| Common Applications | Gas detection, biosensors, environmental monitoring | Optical communication, chemical sensing, biomedical imaging |
| Response Time | Typically rapid, seconds to minutes | Ultra-fast, often microseconds to milliseconds |
| Signal Output | Electrical current or voltage | Optical signal intensity or phase |
| Power Consumption | Low to moderate | Very low, depends on light source |
| Environmental Stability | Can be affected by temperature, humidity | Generally stable, but sensitive to optical noise |
| Cost | Moderate, widely available | Higher initial cost, specialized components |
| Size | Compact, easily miniaturized | Variable, from chip-scale to bulk optics |
Introduction to Electrochemical and Photonic Sensors
Electrochemical sensors detect chemical substances by measuring electrical signals generated from redox reactions, providing high sensitivity for analytes like glucose or gases. Photonic sensors utilize light interactions such as absorption, fluorescence, or refractive index changes to sense environmental or biological parameters with rapid, non-invasive detection. Both sensor types are integral in environmental monitoring, medical diagnostics, and industrial applications due to their distinct detection principles and compatibility with miniaturized devices.
Working Principles of Electrochemical Sensors
Electrochemical sensors operate by converting chemical information into an electrical signal through redox reactions at the sensor's electrode interface. These sensors measure changes in current, voltage, or impedance caused by the target analyte interacting with the electrode surface, enabling precise detection of gases, ions, or biomolecules. Your choice of an electrochemical sensor can deliver highly sensitive and selective measurements, crucial for applications in medical diagnostics and environmental monitoring.
Operating Mechanism of Photonic Sensors
Photonic sensors operate by detecting changes in light properties, such as intensity, phase, wavelength, or polarization, when interacting with a target analyte or environmental condition. These sensors typically use optical fibers, waveguides, or microresonators to guide light and measure variations caused by refractive index changes or absorption. The precise modulation of light signals enables highly sensitive and specific detection in applications like chemical sensing, environmental monitoring, and biomedical diagnostics.
Key Differences Between Electrochemical and Photonic Sensors
Electrochemical sensors detect chemical changes by measuring electrical signals generated from redox reactions, making them highly sensitive to specific analytes in liquids or gases. Photonic sensors rely on the interaction of light with matter, utilizing changes in optical properties such as absorption, reflection, or fluorescence for non-invasive and fast detection. The fundamental difference lies in electrochemical sensors measuring electrical output directly linked to chemical concentration, whereas photonic sensors interpret optical signals influenced by molecular interactions.
Sensitivity and Selectivity Comparison
Electrochemical sensors exhibit high sensitivity by detecting minute changes in electrical signals generated from chemical reactions, making them ideal for trace-level analyte detection. Photonic sensors offer enhanced selectivity through optical properties such as wavelength specificity and refractive index changes, enabling precise identification of target molecules in complex mixtures. Comparing the two, electrochemical sensors excel in sensitivity while photonic sensors provide superior selectivity, critical for applications requiring accurate discrimination of analytes.
Applications in Real-World Environments
Electrochemical sensors are widely used for monitoring air quality, detecting toxic gases, and measuring biomolecules in medical diagnostics, providing precise and cost-effective solutions in real-world environments. Photonic sensors excel in communication networks, environmental monitoring, and industrial automation by offering high sensitivity and real-time data through light-based detection methods. Your choice between these sensors depends on specific application requirements such as accuracy, response time, and environmental conditions.
Advantages of Electrochemical Sensors
Electrochemical sensors offer high sensitivity and selectivity, enabling precise detection of specific analytes in complex environments. Their low power consumption and compact design make them ideal for portable and wearable devices. You benefit from rapid response times and cost-effective manufacturing, facilitating widespread application across healthcare, environmental monitoring, and industrial processes.
Benefits of Photonic Sensor Technology
Photonic sensor technology offers unparalleled sensitivity and rapid response times, enabling precise detection of chemical and biological substances at low concentrations. These sensors are immune to electromagnetic interference, enhancing reliability in environments where electrical noise can compromise electrochemical sensors. Your monitoring systems benefit from the non-invasive, real-time data provided by photonic sensors, improving accuracy and operational efficiency.
Challenges and Limitations of Each Sensor Type
Electrochemical sensors face challenges such as limited selectivity and susceptibility to interference from environmental factors like temperature and humidity, which can affect accuracy and lifespan. Photonic sensors confront limitations including high sensitivity to ambient light conditions and the necessity for precise alignment and calibration, making them less adaptable in dynamic environments. Understanding these constraints can help you choose the suitable sensor type for your specific application requirements.
Future Trends in Sensor Innovation
Future trends in sensor innovation emphasize enhanced sensitivity and real-time monitoring capabilities, with electrochemical sensors advancing through nanomaterial integration to improve selectivity and reduce detection limits. Photonic sensors are evolving by leveraging silicon photonics and integrated optics technologies to enable compact, low-power, and multiplexed detection systems. The convergence of electrochemical and photonic platforms, combined with AI-driven data analytics, is expected to revolutionize smart sensing for healthcare, environmental monitoring, and industrial applications.
Electrochemical vs Photonic Sensor Infographic
 electrown.com
electrown.com