Gas sensors detect and measure the concentration of various gases in the environment using technologies like electrochemical, infrared, or metal oxide semiconductors, while ion-selective electrodes (ISEs) measure the activity of specific ions in liquid solutions through selective membranes. Understanding the differences between these sensors will help you choose the right tool for accurate gas or ion detection; continue reading to explore their applications, advantages, and limitations.
Table of Comparison
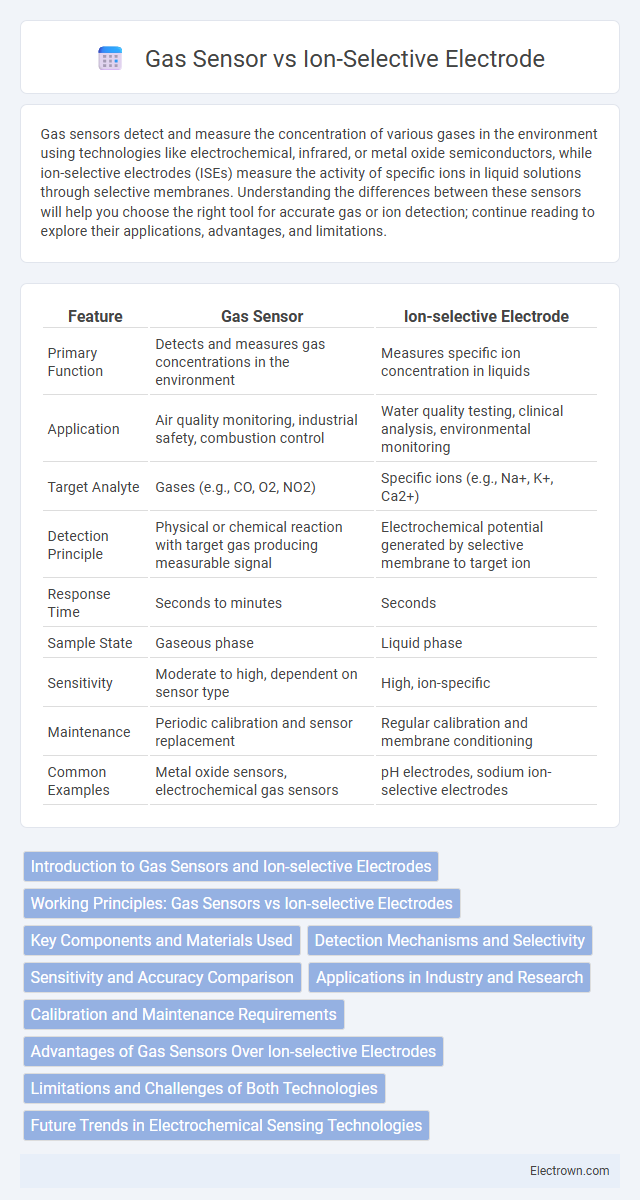
| Feature | Gas Sensor | Ion-selective Electrode |
|---|---|---|
| Primary Function | Detects and measures gas concentrations in the environment | Measures specific ion concentration in liquids |
| Application | Air quality monitoring, industrial safety, combustion control | Water quality testing, clinical analysis, environmental monitoring |
| Target Analyte | Gases (e.g., CO, O2, NO2) | Specific ions (e.g., Na+, K+, Ca2+) |
| Detection Principle | Physical or chemical reaction with target gas producing measurable signal | Electrochemical potential generated by selective membrane to target ion |
| Response Time | Seconds to minutes | Seconds |
| Sample State | Gaseous phase | Liquid phase |
| Sensitivity | Moderate to high, dependent on sensor type | High, ion-specific |
| Maintenance | Periodic calibration and sensor replacement | Regular calibration and membrane conditioning |
| Common Examples | Metal oxide sensors, electrochemical gas sensors | pH electrodes, sodium ion-selective electrodes |
Introduction to Gas Sensors and Ion-selective Electrodes
Gas sensors detect and measure the concentration of gases in the environment, utilizing technologies such as semiconductor, electrochemical, or infrared sensing to provide real-time monitoring of air quality and hazardous gas leaks. Ion-selective electrodes (ISEs) are analytical devices designed to measure the activity of specific ions in solutions, employing selective membranes to achieve high sensitivity and selectivity for ions like sodium, potassium, or calcium. Your choice between gas sensors and ion-selective electrodes depends on whether you need to monitor gaseous substances or ionic concentrations in liquid samples.
Working Principles: Gas Sensors vs Ion-selective Electrodes
Gas sensors detect specific gases by measuring changes in electrical properties or chemical reactions on the sensor surface, often using semiconducting materials or electrochemical cells. Ion-selective electrodes operate based on the selective permeability of a membrane to particular ions, generating a potential difference proportional to the ion concentration in solution. Your choice between these devices depends on whether gas phase detection or ion concentration measurement is required for accurate and reliable monitoring.
Key Components and Materials Used
Gas sensors primarily rely on semiconducting metal oxides like tin dioxide (SnO2) or electrochemical cells with electrodes made from platinum or gold, designed to detect specific gases through changes in electrical resistance or current. Ion-selective electrodes (ISEs) utilize a selective membrane composed of ionophores, polymer matrices such as PVC, and internal electrodes often made of silver/silver chloride to measure specific ion concentrations in solutions. Your choice between these depends on the nature of analytes, where gas sensors target gaseous compounds and ISEs specialize in ion detection in liquid samples.
Detection Mechanisms and Selectivity
Gas sensors detect target gases through changes in electrical conductivity, optical properties, or mass based on chemical reactions at the sensor surface, providing rapid and reversible responses. Ion-selective electrodes (ISEs) operate via selective membrane potentials that arise from specific ion activity differences, enabling highly selective detection of ions in solution. The selectivity of gas sensors depends on material sensitivity to particular gas molecules, while ISEs achieve ion specificity through tailored ionophore membranes that distinguish ions based on size, charge, and chemical affinity.
Sensitivity and Accuracy Comparison
Gas sensors exhibit high sensitivity to specific gas concentrations, often detecting parts-per-million (ppm) levels, making them ideal for real-time environmental monitoring. Ion-selective electrodes provide superior accuracy in measuring ion activity in solutions, with precision often reaching micromolar (uM) detection limits, essential for analytical chemistry applications. The choice between gas sensors and ion-selective electrodes depends on the target analyte and required measurement conditions, where gas sensors excel in gaseous environments and ion-selective electrodes dominate in liquid phase ion detection.
Applications in Industry and Research
Gas sensors are widely used in industrial safety monitoring, environmental analysis, and process control to detect toxic or combustible gases with high sensitivity and rapid response. Ion-selective electrodes (ISEs) are essential in research and industrial applications for precise measurement of ion concentrations in liquids, such as water quality testing, pharmaceuticals, and biochemical assays. Your choice depends on whether gas detection or ion-specific analysis is critical for your application, ensuring accurate monitoring and control in diverse environments.
Calibration and Maintenance Requirements
Gas sensors require frequent calibration using known concentration gas standards to ensure accurate readings, as sensor drift can occur due to environmental factors. Ion-selective electrodes (ISEs) need regular calibration with standard solutions matching the sample matrix to maintain selectivity and sensitivity. Maintenance for gas sensors involves periodic sensor replacement and cleaning, while ISEs require careful membrane conditioning and storage in appropriate solutions to prevent signal degradation.
Advantages of Gas Sensors Over Ion-selective Electrodes
Gas sensors offer rapid, real-time detection of gaseous substances with high sensitivity, making them ideal for monitoring air quality and industrial emissions. These sensors typically require minimal sample preparation and provide a broader detection range compared to ion-selective electrodes, which are limited to specific ionic species in liquid samples. By using a gas sensor, you can achieve more versatile and efficient environmental monitoring, especially in applications where gas detection is critical.
Limitations and Challenges of Both Technologies
Gas sensors face limitations in selectivity and sensitivity, often prone to cross-sensitivity with other gases and environmental factors such as humidity and temperature fluctuations. Ion-selective electrodes encounter challenges including electrode fouling, limited lifespan, and dependence on stable ionic strength for accurate measurements. Your choice between these technologies should consider application-specific requirements, as both have inherent limitations that impact accuracy and reliability in complex sample matrices.
Future Trends in Electrochemical Sensing Technologies
Future trends in electrochemical sensing technologies emphasize enhanced sensitivity, miniaturization, and integration of gas sensors and ion-selective electrodes with IoT devices for real-time monitoring. Advancements in nanomaterials and flexible substrates improve sensor durability and selectivity, enabling precise environmental and healthcare applications. Your access to faster, more reliable electrochemical sensors will drive smarter decision-making in diverse fields, from industrial safety to personalized medicine.
Gas Sensor vs Ion-selective Electrode Infographic
 electrown.com
electrown.com