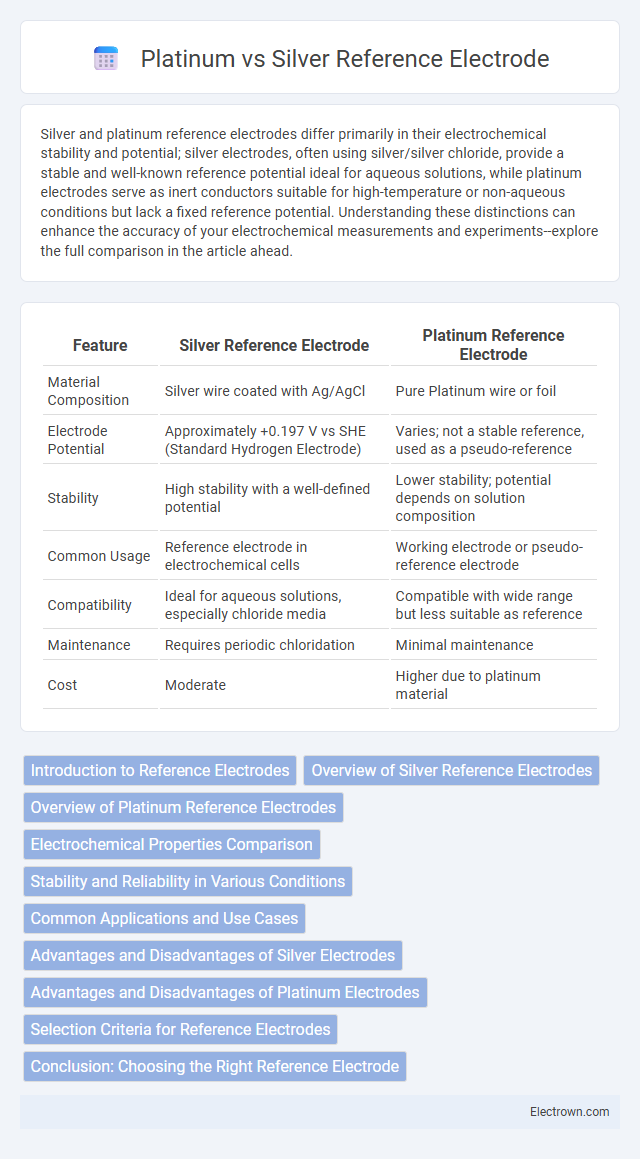
Silver and platinum reference electrodes differ primarily in their electrochemical stability and potential; silver electrodes, often using silver/silver chloride, provide a stable and well-known reference potential ideal for aqueous solutions, while platinum electrodes serve as inert conductors suitable for high-temperature or non-aqueous conditions but lack a fixed reference potential. Understanding these distinctions can enhance the accuracy of your electrochemical measurements and experiments--explore the full comparison in the article ahead.
Table of Comparison
| Feature | Silver Reference Electrode | Platinum Reference Electrode |
|---|---|---|
| Material Composition | Silver wire coated with Ag/AgCl | Pure Platinum wire or foil |
| Electrode Potential | Approximately +0.197 V vs SHE (Standard Hydrogen Electrode) | Varies; not a stable reference, used as a pseudo-reference |
| Stability | High stability with a well-defined potential | Lower stability; potential depends on solution composition |
| Common Usage | Reference electrode in electrochemical cells | Working electrode or pseudo-reference electrode |
| Compatibility | Ideal for aqueous solutions, especially chloride media | Compatible with wide range but less suitable as reference |
| Maintenance | Requires periodic chloridation | Minimal maintenance |
| Cost | Moderate | Higher due to platinum material |
Introduction to Reference Electrodes
Reference electrodes like Silver/Silver Chloride (Ag/AgCl) and Platinum play a crucial role in electrochemical measurements by providing a stable and known potential against which the working electrode's potential can be measured. The Ag/AgCl electrode consists of a silver wire coated with silver chloride immersed in a chloride ion solution, offering a well-defined and reproducible potential, widely used in aqueous systems. Platinum electrodes, often employed as inert and stable reference surfaces, are preferred in non-aqueous or harsh chemical environments where Ag/AgCl electrodes may be unsuitable due to their dependence on chloride ions.
Overview of Silver Reference Electrodes
Silver reference electrodes, commonly utilizing silver/silver chloride (Ag/AgCl), provide stable and reliable potential in electrochemical measurements. Their wide use in laboratory and field environments stems from low cost, ease of preparation, and consistent reference potential of approximately +0.197 V versus the standard hydrogen electrode (SHE). You can expect high reproducibility and low maintenance when using silver reference electrodes in aqueous solutions.
Overview of Platinum Reference Electrodes
Platinum reference electrodes offer exceptional chemical inertness and stability in various electrochemical applications, making them suitable for harsh or aggressive environments. Their high resistance to corrosion and wide potential range provide reliable and reproducible measurements compared to silver-based references. You can depend on platinum electrodes for accuracy in experiments involving strong oxidizing conditions or where long-term electrode durability is critical.
Electrochemical Properties Comparison
Silver reference electrodes exhibit a stable and well-defined electrochemical potential, typically around 0.197 V versus the standard hydrogen electrode (SHE), making them suitable for chloride ion environments. Platinum electrodes provide a wider potential window and greater inertness, offering excellent stability for redox reactions in various electrolyte solutions, including acidic and neutral media. The choice between silver and platinum reference electrodes depends on the specific electrochemical system requirements, such as potential precision, chemical resistance, and compatibility with different solvents.
Stability and Reliability in Various Conditions
Silver reference electrodes exhibit good stability in neutral and mildly acidic environments, making them suitable for general electrochemical measurements. Platinum reference electrodes offer superior reliability and maintain stable potentials under a wider range of conditions, including highly oxidizing or reducing environments and elevated temperatures. The chemical inertness of platinum ensures minimal degradation, enhancing long-term reproducibility compared to silver electrodes.
Common Applications and Use Cases
Silver reference electrodes are commonly used in electrochemical sensors, pH measurement, and corrosion testing due to their stable potential in chloride-containing solutions. Platinum reference electrodes find frequent applications in high-temperature environments, fuel cells, and oxygen reduction reactions because of their inertness and catalytic properties. Both types are essential in laboratory and industrial electrochemical experiments, with silver electrodes favored for aqueous systems and platinum electrodes preferred for durable and harsh conditions.
Advantages and Disadvantages of Silver Electrodes
Silver reference electrodes offer advantages such as low cost, ease of fabrication, and stable potential in chloride-containing solutions, making them suitable for routine electrochemical measurements. However, they exhibit disadvantages including sensitivity to sulfide contamination, potential drift over time, and limited chemical compatibility in non-chloride or high-temperature environments. These factors restrict their applicability compared to more robust platinum electrodes, especially in long-term or harsh condition applications.
Advantages and Disadvantages of Platinum Electrodes
Platinum reference electrodes offer high chemical stability and excellent resistance to corrosion, making them ideal for long-term electrochemical measurements. Their superior conductivity and inert nature enhance response accuracy and reproducibility, but the high cost and susceptibility to surface contamination can limit widespread use. Your choice of a platinum electrode should balance its durability and performance against budget and maintenance requirements.
Selection Criteria for Reference Electrodes
Selection criteria for Silver vs Platinum reference electrodes depend on factors such as chemical stability, potential reproducibility, and application environment. Silver reference electrodes, especially Ag/AgCl types, offer stable and well-defined potentials in aqueous environments and are ideal for biological or environmental sensing due to low toxicity. Platinum electrodes provide inertness and durability, making them suitable for harsh chemical conditions or high-temperature applications, but may lack the defined potential stability required for precise measurements in your electrochemical experiments.
Conclusion: Choosing the Right Reference Electrode
Selecting the appropriate reference electrode depends on the specific electrochemical application, with silver reference electrodes favored for their stability in chloride-containing solutions due to their Ag/AgCl system, while platinum electrodes offer inertness and durability in a wider range of environments without chloride interference. Silver reference electrodes provide accurate and reproducible potentials in aqueous systems, especially in physiological or environmental testing. Platinum reference electrodes are preferable for non-aqueous or harsh chemical conditions requiring resistance to corrosion and catalytic properties.
Silver vs Platinum Reference Electrode Infographic
 electrown.com
electrown.com