Implantable devices offer continuous monitoring and precise data collection directly from within the body, while external devices provide non-invasive, easily accessible options for managing your health. Explore the rest of the article to understand which option best suits your lifestyle and medical needs.
Table of Comparison
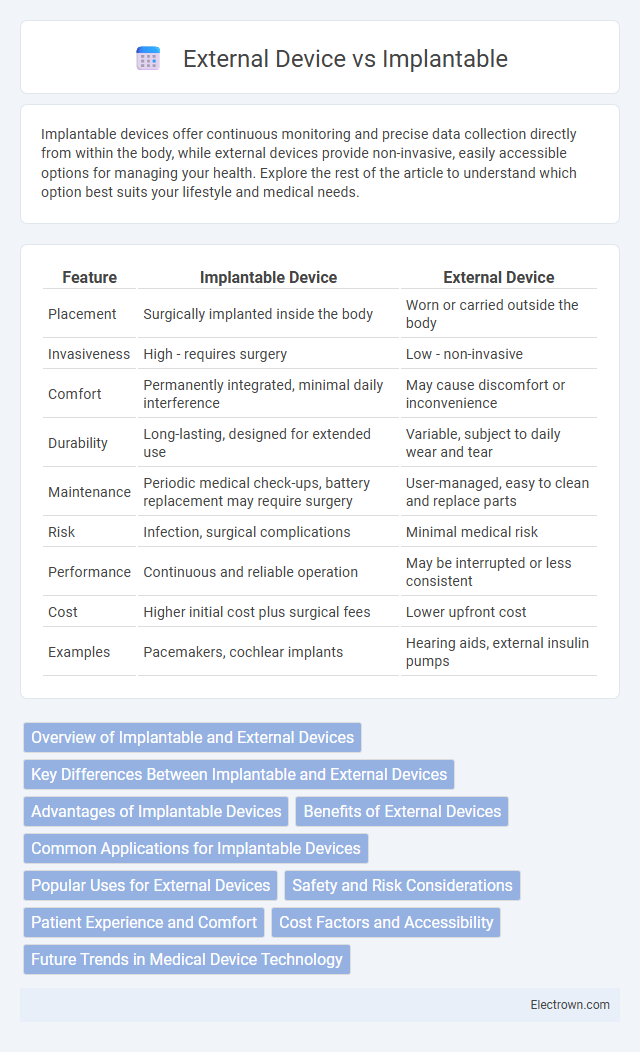
| Feature | Implantable Device | External Device |
|---|---|---|
| Placement | Surgically implanted inside the body | Worn or carried outside the body |
| Invasiveness | High - requires surgery | Low - non-invasive |
| Comfort | Permanently integrated, minimal daily interference | May cause discomfort or inconvenience |
| Durability | Long-lasting, designed for extended use | Variable, subject to daily wear and tear |
| Maintenance | Periodic medical check-ups, battery replacement may require surgery | User-managed, easy to clean and replace parts |
| Risk | Infection, surgical complications | Minimal medical risk |
| Performance | Continuous and reliable operation | May be interrupted or less consistent |
| Cost | Higher initial cost plus surgical fees | Lower upfront cost |
| Examples | Pacemakers, cochlear implants | Hearing aids, external insulin pumps |
Overview of Implantable and External Devices
Implantable devices, such as pacemakers and cochlear implants, are surgically placed inside the body to provide continuous monitoring or therapy with minimal external interference. External devices, including wearable glucose monitors and hearing aids, operate outside the body and offer easier access for maintenance and adjustments but may be less discreet. Both device types serve critical roles in medical treatment, balancing invasiveness, convenience, and functionality based on patient needs and clinical objectives.
Key Differences Between Implantable and External Devices
Implantable devices are surgically placed inside the body, offering continuous, discreet monitoring or therapy with minimal user intervention, while external devices remain outside the body and require regular handling or attachment. Implantable devices provide greater stability and reduce infection risk compared to external devices, which are often easier to remove and upgrade without surgery. Your choice depends on factors like long-term use, lifestyle, and the level of device management you prefer.
Advantages of Implantable Devices
Implantable devices offer continuous, real-time monitoring and therapy without the inconvenience of external components, enhancing patient comfort and compliance. These devices reduce the risk of infection and device displacement compared to external counterparts by being fully enclosed within the body. Your treatment becomes more seamless and reliable with implantable devices, providing improved long-term outcomes and quality of life.
Benefits of External Devices
External medical devices offer non-invasive application, minimizing surgical risks and allowing easier adjustments or replacements compared to implantable options. They provide greater accessibility for maintenance and monitoring, enhancing patient compliance and real-time management. External devices also enable flexible use across different conditions without permanent alteration to the body, making them suitable for diverse therapeutic needs.
Common Applications for Implantable Devices
Implantable devices are commonly used in medical applications such as cardiac pacemakers, cochlear implants, and insulin pumps, providing continuous, real-time therapeutic support directly within the body. These devices offer enhanced precision and reliability compared to external alternatives, making them ideal for managing chronic conditions like heart arrhythmias and hearing loss. Your healthcare team may recommend implantable solutions when long-term, consistent intervention is essential for improving quality of life.
Popular Uses for External Devices
External devices are commonly utilized for hearing aids, continuous glucose monitors, and wearable cardiac monitors due to their non-invasive nature and ease of upgrading. These devices enable real-time data collection and communication with smartphones or healthcare providers, enhancing patient monitoring and management. Their popularity is driven by convenience, cost-effectiveness, and the ability to customize settings without surgical intervention.
Safety and Risk Considerations
Implantable devices present increased safety concerns due to the risk of infection, immune response, and surgical complications, necessitating rigorous preoperative assessment and sterile implantation procedures. External devices minimize invasive risks but may expose users to skin irritation, device malfunctions, and security vulnerabilities from external environmental factors. Thorough risk-benefit analysis and continuous monitoring protocols are essential for both device types to ensure patient safety and address potential adverse effects.
Patient Experience and Comfort
Implantable devices offer patients enhanced comfort by eliminating external components, reducing the risk of device displacement and daily maintenance. External devices provide easier access for adjustments and monitoring but may cause skin irritation, discomfort, and social self-consciousness due to visible hardware. Patient experience varies widely depending on individual lifestyle, with implantable options often preferred for long-term convenience and discreetness.
Cost Factors and Accessibility
Implantable devices typically incur higher upfront costs due to surgery and specialized equipment, while external devices offer more affordable and flexible options with lower initial expenses. Insurance coverage and healthcare infrastructure significantly influence accessibility, with implantable devices often limited to patients with comprehensive insurance or access to specialized medical centers. The ongoing maintenance and replacement costs of external devices can accumulate over time, potentially offsetting their initial affordability compared to implantable alternatives.
Future Trends in Medical Device Technology
Future trends in medical device technology emphasize the development of smarter implantable devices with enhanced biocompatibility and real-time health monitoring capabilities, driven by advancements in nanotechnology and AI integration. External devices are evolving with improved wireless connectivity, portability, and user-friendly interfaces to provide seamless remote patient management. Your healthcare outcomes can significantly benefit as these innovations enable personalized treatment and continuous, accurate data collection for proactive medical interventions.
Implantable vs External Device Infographic
 electrown.com
electrown.com