Platinum and iridium electrodes differ significantly in conductivity, durability, and corrosion resistance, with platinum offering excellent electrical conductivity and iridium providing superior corrosion resistance under extreme conditions. Discover the key factors that will help you choose the right electrode for your specific application in the detailed comparison ahead.
Table of Comparison
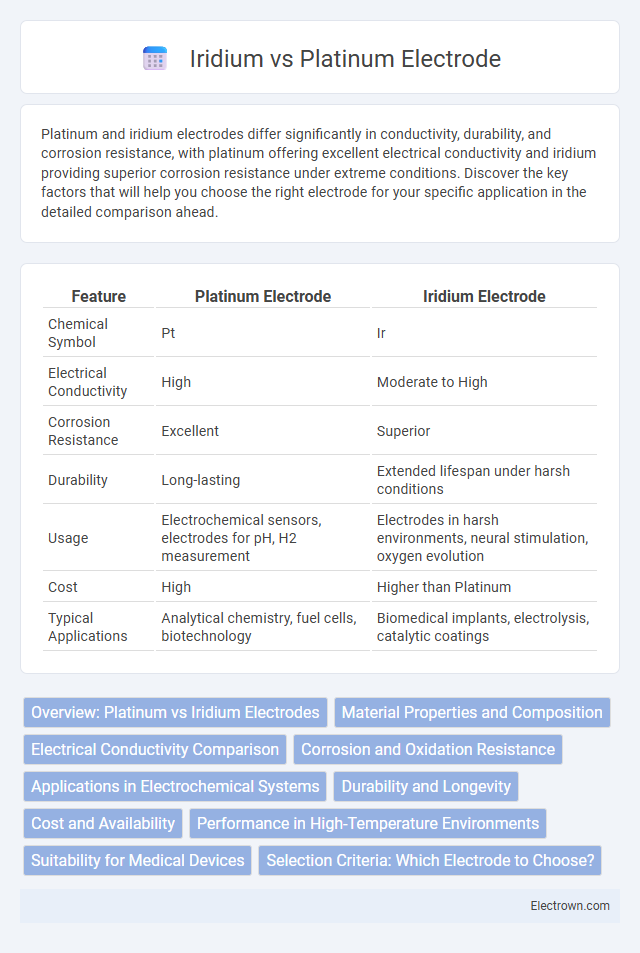
| Feature | Platinum Electrode | Iridium Electrode |
|---|---|---|
| Chemical Symbol | Pt | Ir |
| Electrical Conductivity | High | Moderate to High |
| Corrosion Resistance | Excellent | Superior |
| Durability | Long-lasting | Extended lifespan under harsh conditions |
| Usage | Electrochemical sensors, electrodes for pH, H2 measurement | Electrodes in harsh environments, neural stimulation, oxygen evolution |
| Cost | High | Higher than Platinum |
| Typical Applications | Analytical chemistry, fuel cells, biotechnology | Biomedical implants, electrolysis, catalytic coatings |
Overview: Platinum vs Iridium Electrodes
Platinum and iridium electrodes are widely used in electrochemical applications due to their excellent conductivity and corrosion resistance. Platinum electrodes offer superior chemical stability and are commonly employed in sensors, fuel cells, and catalysis, while iridium electrodes provide enhanced durability and higher resistance to oxidation, making them suitable for harsh environments and long-term use. Both metals serve critical roles in electrolysis and biomedical devices, with the selection depending on specific performance requirements and environmental conditions.
Material Properties and Composition
Platinum electrodes feature a dense, malleable metal known for excellent corrosion resistance and electrical conductivity, primarily composed of pure platinum or platinum alloys. Iridium electrodes consist mainly of iridium, a rare, extremely hard metal with a higher melting point and superior chemical stability under extreme conditions compared to platinum. The distinct material properties influence their applications, where platinum's softness suits catalytic processes, while iridium's durability ensures longevity in high-stress electrochemical environments.
Electrical Conductivity Comparison
Platinum and iridium electrodes demonstrate distinct differences in electrical conductivity, with platinum exhibiting higher electrical conductivity due to its superior electron mobility and lower resistivity of approximately 10.6 mO*cm compared to iridium's 5.3 mO*cm, which is notable despite iridium's higher resistivity because of its robust catalytic properties. The intrinsic conductivity of platinum facilitates efficient current flow in electrochemical applications, making it preferable for sensor electrodes and catalytic converters. Iridium electrodes, although slightly less conductive, offer enhanced corrosion resistance and stability in harsh environments, which compensates in applications requiring durability over pure conductivity.
Corrosion and Oxidation Resistance
Platinum electrodes exhibit exceptional corrosion resistance due to their inert nature, making them highly durable in harsh chemical environments. Iridium electrodes, while slightly less resistant to corrosion than platinum, offer superior oxidation resistance at elevated temperatures, which enhances their performance in high-temperature applications. Your choice between these electrodes should consider the specific environmental conditions, with platinum favored for acidic or chloride-rich environments and iridium preferred for oxidative or thermal stress conditions.
Applications in Electrochemical Systems
Platinum electrodes are widely used in electrochemical systems for their excellent catalytic activity, corrosion resistance, and stability in acidic environments, making them ideal for fuel cells and electrolysis processes. Iridium electrodes offer superior performance in harsh conditions, such as oxygen evolution reactions in water splitting, due to their exceptional durability and resistance to oxidation. Both materials are critical in sensors, electroplating, and electrochemical synthesis, with choice depending on specific application demands for stability and catalytic efficiency.
Durability and Longevity
Platinum electrodes exhibit exceptional durability due to their high resistance to corrosion and stable chemical properties, making them suitable for prolonged use in various electrochemical applications. Iridium electrodes, while slightly less corrosion-resistant than platinum, offer outstanding longevity under extreme conditions, including high temperatures and acidic environments, due to their robust mechanical strength. Both materials provide long service life, but platinum is often preferred for general durability, whereas iridium excels in specialized, harsh operational settings.
Cost and Availability
Iridium electrodes generally cost more than platinum due to the rarity and complex extraction process of iridium, which is one of the rarest elements in the Earth's crust. Platinum, while also expensive, benefits from greater industrial demand and more established supply chains, making it relatively more available and affordable. The limited availability and high cost of iridium often restrict its use to specialized applications where its superior corrosion resistance justifies the premium.
Performance in High-Temperature Environments
Platinum electrodes maintain excellent stability and conductivity at high temperatures, making them suitable for applications requiring consistent performance in environments exceeding 1000degC. Iridium electrodes exhibit superior resistance to thermal degradation and oxidation, offering enhanced durability in extreme high-temperature conditions often found in industrial processes. Your choice between platinum and iridium electrodes should consider the operating temperature range and the need for long-term stability under thermal stress.
Suitability for Medical Devices
Platinum electrodes offer excellent biocompatibility, corrosion resistance, and electrical conductivity, making them highly suitable for long-term implantable medical devices such as pacemakers and neural stimulators. Iridium electrodes, often used in neural recording and stimulation, provide superior charge injection capacity and durability under repetitive pulsing, making them ideal for advanced neuroprosthetics and deep brain stimulation devices. Both metals meet rigorous medical standards, but platinum is preferred for stable, chronic applications while iridium excels in high-performance neural interfaces.
Selection Criteria: Which Electrode to Choose?
Platinum electrodes are preferred for general electrochemical applications due to their excellent conductivity, chemical stability, and cost-effectiveness, especially in environments where corrosion resistance is vital. Iridium electrodes offer superior durability and performance in highly aggressive or high-temperature conditions, making them ideal for industrial processes requiring extended electrode lifespan. The choice depends on specific factors such as chemical environment, temperature, required electrode longevity, and budget constraints, with platinum favored for versatility and iridium for extreme operational demands.
Platinum vs Iridium Electrode Infographic
 electrown.com
electrown.com