TIRF microscopy offers superior surface sensitivity and high-contrast imaging by selectively exciting fluorophores near the cell membrane, while confocal microscopy provides detailed optical sectioning and 3D imaging deep within samples. Discover how these advanced imaging techniques can enhance your research by reading the rest of the article.
Table of Comparison
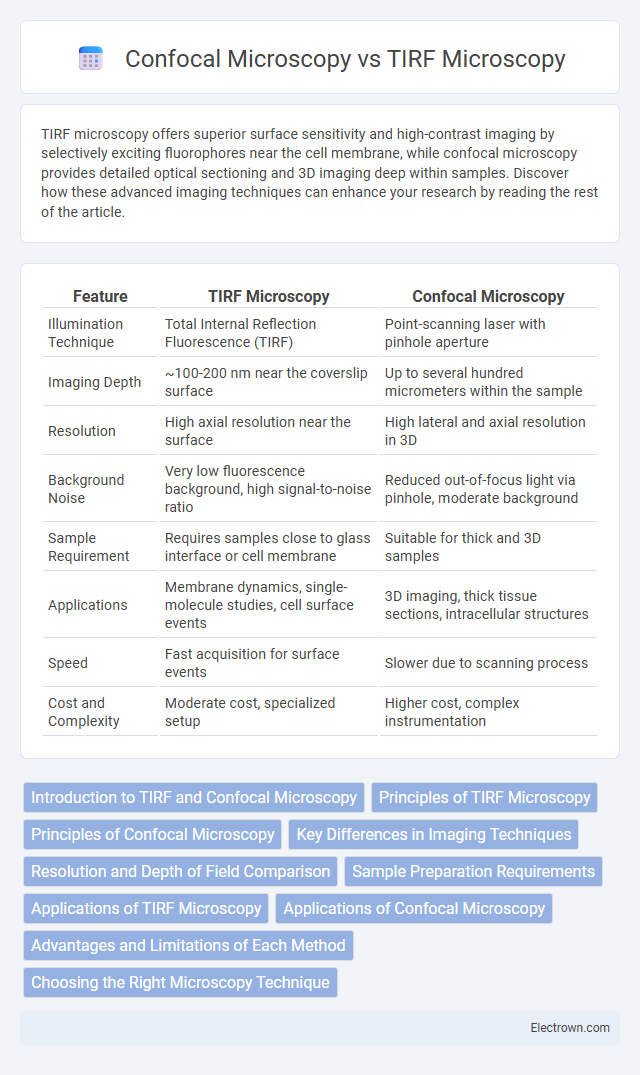
| Feature | TIRF Microscopy | Confocal Microscopy |
|---|---|---|
| Illumination Technique | Total Internal Reflection Fluorescence (TIRF) | Point-scanning laser with pinhole aperture |
| Imaging Depth | ~100-200 nm near the coverslip surface | Up to several hundred micrometers within the sample |
| Resolution | High axial resolution near the surface | High lateral and axial resolution in 3D |
| Background Noise | Very low fluorescence background, high signal-to-noise ratio | Reduced out-of-focus light via pinhole, moderate background |
| Sample Requirement | Requires samples close to glass interface or cell membrane | Suitable for thick and 3D samples |
| Applications | Membrane dynamics, single-molecule studies, cell surface events | 3D imaging, thick tissue sections, intracellular structures |
| Speed | Fast acquisition for surface events | Slower due to scanning process |
| Cost and Complexity | Moderate cost, specialized setup | Higher cost, complex instrumentation |
Introduction to TIRF and Confocal Microscopy
TIRF microscopy exploits the phenomenon of total internal reflection to selectively illuminate and excite fluorophores within a shallow evanescent field, typically less than 200 nm from the glass-cell interface, enabling high-contrast imaging of membrane and surface events. Confocal microscopy utilizes point illumination and a spatial pinhole to eliminate out-of-focus light, achieving optical sectioning and three-dimensional reconstruction of specimens with enhanced resolution and depth penetration. Both techniques are fundamental in fluorescence imaging but differ in their illumination strategies and applications, with TIRF excelling in surface-sensitive studies and confocal microscopy providing volumetric imaging of thicker samples.
Principles of TIRF Microscopy
TIRF microscopy utilizes the evanescent wave generated by total internal reflection at the interface between glass and aqueous sample, exciting fluorophores within approximately 100-200 nm of the specimen surface, enabling high-contrast imaging of membrane-associated processes. Unlike confocal microscopy, which employs point illumination and pinhole spatial filtering to achieve optical sectioning throughout the specimen depth, TIRF selectively excites fluorophores only near the interface, minimizing background fluorescence and photobleaching. This surface-restricted excitation allows TIRF microscopy to achieve superior signal-to-noise ratios for studying cell membrane dynamics, protein interactions, and single-molecule events compared to deeper optical sectioning in confocal microscopy.
Principles of Confocal Microscopy
Confocal microscopy employs point illumination and a spatial pinhole to eliminate out-of-focus light, enhancing optical resolution and contrast in thick specimens. By scanning the specimen with focused laser beams, it constructs high-resolution, three-dimensional images through optical sectioning. This imaging technique is essential for analyzing cellular structures and dynamic processes with precise spatial localization.
Key Differences in Imaging Techniques
TIRF microscopy exploits an evanescent wave to selectively illuminate and excite fluorophores within a 100-200 nm region near the cell membrane, providing exceptional surface sensitivity and minimal background fluorescence. Confocal microscopy employs point illumination and a spatial pinhole to eliminate out-of-focus light from deeper within the specimen, enabling high-resolution optical sectioning and three-dimensional imaging. While TIRF is ideal for studying membrane-associated processes, confocal microscopy excels in imaging thick samples and capturing detailed intracellular structures.
Resolution and Depth of Field Comparison
TIRF microscopy offers superior axial resolution with an evanescent wave penetrating only about 100-200 nm, enabling precise imaging of cell membranes and surface structures. Confocal microscopy provides moderate axial resolution, typically around 500 nm, with a greater depth of field, allowing optical sectioning and imaging up to several hundred micrometers inside specimens. The limited penetration depth of TIRF is ideal for studying phenomena at or near the cell surface, while confocal microscopy excels in imaging thicker samples with three-dimensional resolution.
Sample Preparation Requirements
TIRF microscopy requires samples to be placed on a specialized glass coverslip with an appropriate refractive index to exploit the evanescent wave for selective surface imaging, which limits the imaging to near-membrane regions. Confocal microscopy allows more flexible sample preparation as it can image thicker specimens by optical sectioning without specialized substrates. Your choice depends on the need for surface-specific imaging in TIRF or volumetric imaging capabilities offered by confocal microscopy.
Applications of TIRF Microscopy
TIRF microscopy excels in imaging near-membrane events with high axial resolution, making it ideal for studying cell surface interactions, membrane dynamics, and single-molecule fluorescence near the plasma membrane. This technique enables real-time observation of processes such as endocytosis, receptor-ligand binding, and cytoskeletal organization at the cell periphery. In contrast to confocal microscopy, TIRF's selective excitation minimizes background fluorescence, enhancing sensitivity in applications like total internal reflection fluorescence single-molecule tracking and live-cell membrane protein analysis.
Applications of Confocal Microscopy
Confocal microscopy is widely used in cell biology for high-resolution imaging of thick specimens, enabling detailed visualization of cellular structures, protein localization, and intracellular processes. It excels in 3D reconstruction of tissues, live cell imaging, and fluorescence studies, providing optical sectioning that reduces background noise and enhances image contrast. These capabilities make confocal microscopy essential for applications such as neuroscience, developmental biology, and pathology research.
Advantages and Limitations of Each Method
TIRF microscopy offers superior imaging of cell membranes and surface events with high signal-to-noise ratio due to its shallow evanescent wave excitation, but it is limited to thin samples near the glass interface. Confocal microscopy provides optical sectioning and deep tissue imaging with improved resolution in thicker specimens, yet it involves higher phototoxicity and slower image acquisition. Your choice depends on whether you prioritize surface-specific sensitivity or three-dimensional imaging capability.
Choosing the Right Microscopy Technique
TIRF microscopy excels in studying events at or near the cell membrane with high axial resolution and minimal background fluorescence, making it ideal for investigating processes like membrane protein interactions and vesicle trafficking. Confocal microscopy offers deeper tissue imaging and optical sectioning, providing detailed 3D reconstructions suitable for thicker samples and intracellular structures. Choosing the right microscopy technique depends on your specific imaging needs, such as the depth of observation and resolution required for your biological questions.
TIRF microscopy vs Confocal microscopy Infographic
 electrown.com
electrown.com