The Landauer principle establishes a fundamental thermodynamic lower bound on the amount of energy required to erase one bit of information, directly connecting information theory to physical thermodynamics. Explore the following sections to understand how this principle contrasts with the broader thermodynamic limits affecting computation and energy efficiency, and what it means for Your future technologies.
Table of Comparison
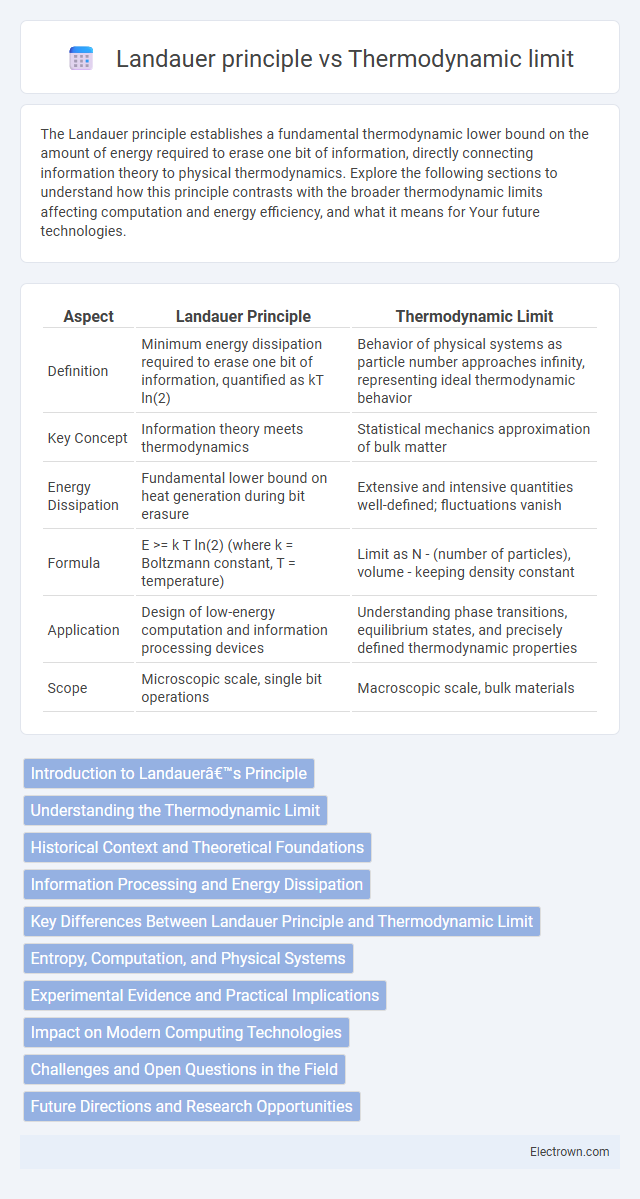
| Aspect | Landauer Principle | Thermodynamic Limit |
|---|---|---|
| Definition | Minimum energy dissipation required to erase one bit of information, quantified as kT ln(2) | Behavior of physical systems as particle number approaches infinity, representing ideal thermodynamic behavior |
| Key Concept | Information theory meets thermodynamics | Statistical mechanics approximation of bulk matter |
| Energy Dissipation | Fundamental lower bound on heat generation during bit erasure | Extensive and intensive quantities well-defined; fluctuations vanish |
| Formula | E >= k T ln(2) (where k = Boltzmann constant, T = temperature) | Limit as N - (number of particles), volume - keeping density constant |
| Application | Design of low-energy computation and information processing devices | Understanding phase transitions, equilibrium states, and precisely defined thermodynamic properties |
| Scope | Microscopic scale, single bit operations | Macroscopic scale, bulk materials |
Introduction to Landauer’s Principle
Landauer's Principle establishes a fundamental limit on the minimum energy required to erase one bit of information, quantifying the thermodynamic cost of computation as at least kT ln(2), where k is Boltzmann's constant and T is the temperature of the computing environment. This principle bridges information theory and thermodynamics by demonstrating that information processing inherently involves physical energy dissipation. Understanding Landauer's Principle helps you optimize computing systems by acknowledging the irreducible thermodynamic limits of energy consumption during information erasure.
Understanding the Thermodynamic Limit
The thermodynamic limit describes the behavior of systems as the number of particles approaches infinity, ensuring that macroscopic properties become well-defined and fluctuations negligible. Landauer's principle ties information processing to physical entropy, establishing a fundamental energy cost for bit erasure, which becomes more apparent when considering finite systems outside the thermodynamic limit. Understanding the thermodynamic limit helps clarify how minimal energy dissipation approaches but never violates Landauer's bound in large-scale computational processes.
Historical Context and Theoretical Foundations
Landauer's principle, formulated in 1961, establishes a fundamental thermodynamic limit by quantifying the minimum energy dissipation required to erase one bit of information, linking information theory with physical thermodynamics. This principle emerged from Rolf Landauer's insight that information is physical, refuting the notion that logical irreversibility can be achieved without thermodynamic cost and grounding computation within the laws of thermodynamics. The theoretical foundation is deeply rooted in the second law of thermodynamics, emphasizing entropy reduction during bit erasure and setting a lower bound on heat generation equal to kT ln 2 per bit, where k is Boltzmann's constant and T the temperature.
Information Processing and Energy Dissipation
The Landauer principle sets a fundamental lower bound on the energy dissipation required for irreversible information processing, quantifying this minimum as \( k_B T \ln 2 \) per bit erased, where \( k_B \) is Boltzmann's constant and \( T \) is the temperature of the thermal reservoir. This principle bridges information theory and thermodynamics by linking logic operations to physical laws, highlighting that any computation involving bit erasure generates unavoidable heat. While the thermodynamic limit defines the ultimate efficiency constraints for energy conversions, Landauer's principle specifically addresses the intrinsic energy cost of computation, marking a key threshold for minimizing dissipation in information processing devices.
Key Differences Between Landauer Principle and Thermodynamic Limit
The Landauer Principle establishes a fundamental minimum energy dissipation, quantified as kT ln(2) per bit erasure, linking information theory with thermodynamics. In contrast, the thermodynamic limit concerns the behavior of macroscopic systems as particle number approaches infinity, focusing on bulk properties rather than minimal energy costs per operation. Key differences lie in their scope: Landauer's Principle addresses microscopic information processing constraints, while the thermodynamic limit pertains to large-scale system equilibrium and phase transitions.
Entropy, Computation, and Physical Systems
Landauer's principle establishes a fundamental link between information theory and thermodynamics by quantifying the minimum entropy increase during the irreversible erasure of one bit of information, which is k_B ln 2 per bit. This principle sets a physical lower bound on the energy dissipation in any computational process, implying that computation in physical systems cannot be entirely free from thermodynamic costs. Understanding this relationship helps optimize your computational devices to approach the thermodynamic limit, minimizing entropy production while maintaining effective information processing.
Experimental Evidence and Practical Implications
Experimental evidence supporting the Landauer principle demonstrates that information erasure incurs a minimum heat dissipation close to kT ln 2 per bit, confirming a fundamental thermodynamic limit on computation. Recent experiments employing ultra-cold atoms and nanoscale electronic circuits have measured energy dissipation at or near this theoretical bound, reinforcing the principle's validity in real physical systems. Practical implications emphasize the design of ultra-low-power computing devices, where approaching the Landauer limit could drastically reduce energy consumption in information processing technologies.
Impact on Modern Computing Technologies
Landauer's principle establishes a fundamental thermodynamic limit on the minimum possible energy dissipation during information erasure, quantifying it as kT ln 2 per bit operation, which directly influences the design of low-power computing devices. As modern computing technologies approach this thermodynamic limit, innovations in reversible computing and quantum information processing are increasingly critical to overcoming energy efficiency barriers. The alignment of practical hardware with theoretical limits drives advancements in minimizing heat generation, enabling more sustainable and scalable computational architectures.
Challenges and Open Questions in the Field
Landauer principle, which establishes a fundamental thermodynamic cost for information erasure at kT ln 2 per bit, faces challenges in reconciling practical energy dissipation with thermodynamic limit predictions in nanoscale systems. Open questions remain about how quantum effects, error rates, and non-equilibrium processes influence the minimum energy cost and whether true reversibility can be approached experimentally. Addressing these issues requires advanced studies in quantum thermodynamics, stochastic thermodynamics, and the development of noise-resilient computing architectures.
Future Directions and Research Opportunities
Future research on the Landauer principle seeks to bridge its theoretical foundations with practical implementations in nanoscale computing and quantum information processing. Exploring thermodynamic limits in irreversible computations offers opportunities to minimize energy dissipation below current benchmarks, enhancing energy-efficient technologies. Advancements in materials science and experimental techniques aim to validate and refine the principle's application at quantum scales, pushing boundaries of fundamental thermodynamics and computational physics.
Landauer principle vs thermodynamic limit Infographic
 electrown.com
electrown.com