Silver-Silver Chloride electrodes offer stable and reproducible reference potentials with low noise, making them ideal for precise electrochemical measurements, while gold electrodes provide excellent conductivity and chemical inertness suitable for bio-sensing and corrosion studies. Discover how to choose the best electrode for Your specific application by reading the rest of the article.
Table of Comparison
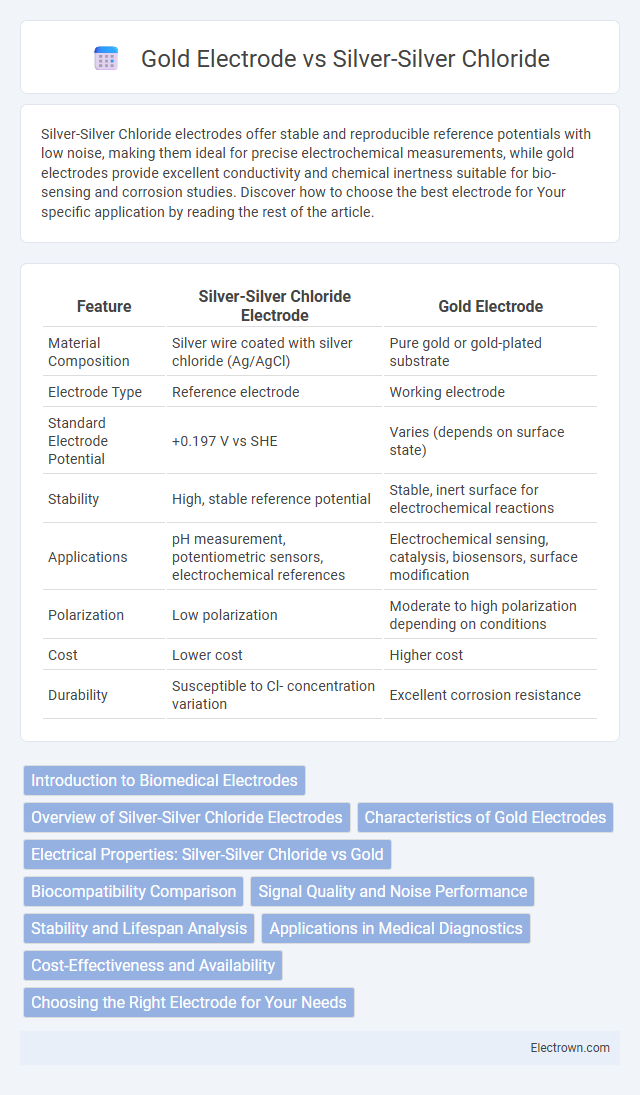
| Feature | Silver-Silver Chloride Electrode | Gold Electrode |
|---|---|---|
| Material Composition | Silver wire coated with silver chloride (Ag/AgCl) | Pure gold or gold-plated substrate |
| Electrode Type | Reference electrode | Working electrode |
| Standard Electrode Potential | +0.197 V vs SHE | Varies (depends on surface state) |
| Stability | High, stable reference potential | Stable, inert surface for electrochemical reactions |
| Applications | pH measurement, potentiometric sensors, electrochemical references | Electrochemical sensing, catalysis, biosensors, surface modification |
| Polarization | Low polarization | Moderate to high polarization depending on conditions |
| Cost | Lower cost | Higher cost |
| Durability | Susceptible to Cl- concentration variation | Excellent corrosion resistance |
Introduction to Biomedical Electrodes
Silver-Silver Chloride electrodes offer stable, low-noise, and reproducible signals, making them ideal for precise biomedical measurements like ECG and EEG. Gold electrodes provide excellent biocompatibility and corrosion resistance, allowing reliable long-term recordings on the skin or implanted devices. Your choice depends on application-specific requirements such as signal quality, longevity, and tissue compatibility in biomedical monitoring.
Overview of Silver-Silver Chloride Electrodes
Silver-silver chloride (Ag/AgCl) electrodes are widely used as reference electrodes due to their stable and reproducible potential in aqueous solutions, making them ideal for accurate electrochemical measurements. Constructed by coating a silver wire with a layer of silver chloride, these electrodes offer low impedance and minimal polarization, which enhances measurement precision in pH sensing, potentiometry, and other electrochemical techniques. Your choice of Ag/AgCl electrodes can significantly improve signal stability and reliability compared to gold electrodes, which often serve as inert working electrodes but lack the inherent reference properties of silver-silver chloride systems.
Characteristics of Gold Electrodes
Gold electrodes exhibit excellent chemical inertness and biocompatibility, making them ideal for sensitive electrochemical measurements and biosensing applications. Their high electrical conductivity and resistance to corrosion provide stable and reproducible results in complex sample matrices. You benefit from their durability and stable electrode potentials, which ensure precise analytical performance in various environments.
Electrical Properties: Silver-Silver Chloride vs Gold
Silver-silver chloride electrodes exhibit lower impedance and higher stability in physiological environments compared to gold electrodes, making them ideal for accurate bioelectric signal measurements. Gold electrodes offer excellent conductivity and biocompatibility but typically present higher interfacial resistance and less consistent reference potentials than silver-silver chloride. Your choice depends on the required balance between signal stability and conductivity in applications like electrocardiography or neural recording.
Biocompatibility Comparison
Silver-Silver Chloride electrodes exhibit excellent biocompatibility due to their stable electrochemical properties and minimal tissue irritation, making them ideal for long-term biomedical monitoring. Gold electrodes also provide good biocompatibility with resistance to corrosion and biofouling, often preferred for applications requiring inertness and surface modification. Your choice depends on the specific biomedical application, weighing silver-silver chloride's superior signal stability against gold's chemical inertness and flexibility in surface functionalization.
Signal Quality and Noise Performance
Silver-Silver Chloride electrodes provide superior signal quality due to their stable half-cell potential and low impedance interface, minimizing drift and baseline noise during measurements. Gold electrodes, while highly conductive and biocompatible, tend to exhibit higher noise levels caused by surface oxidation and varying electrochemical reactions. Your choice between these electrodes should consider the critical need for low noise and precise signal detection in applications like electrophysiology or biosensing.
Stability and Lifespan Analysis
Silver-Silver Chloride electrodes offer superior stability and a longer lifespan in aqueous environments due to their resistance to corrosion and stable reference potential. Gold electrodes, while biocompatible and corrosion-resistant, may experience surface degradation and signal drift over extended use. Your choice depends on the specific application requirements, balancing stability needs with electrode longevity.
Applications in Medical Diagnostics
Silver-Silver Chloride electrodes are widely used in medical diagnostics due to their stable and low-noise reference potential, making them ideal for electrocardiograms (ECG) and electroencephalograms (EEG) where accurate bioelectric signal detection is crucial. Gold electrodes offer excellent biocompatibility and corrosion resistance, which is beneficial in implantable sensors and biosensors for continuous monitoring of glucose, pH, and other analytes. Both electrode types play critical roles in wearable health devices and point-of-care testing, leveraging their unique electrochemical properties to enable sensitive and reliable medical diagnostics.
Cost-Effectiveness and Availability
Silver-Silver Chloride electrodes offer greater cost-effectiveness due to the lower price and widespread availability of silver compared to gold. Their accessibility ensures consistent supply for various electrochemical applications, making them ideal for budget-conscious projects. Your choice may favor silver-silver chloride when balancing performance with affordability and easy procurement.
Choosing the Right Electrode for Your Needs
Silver-silver chloride (Ag/AgCl) electrodes provide stable reference potentials and low noise, making them ideal for precise pH measurements and electrophysiological applications. Gold electrodes offer excellent conductivity and chemical inertness, suitable for biosensors and electrochemical sensing where biocompatibility and surface modification are critical. Selecting between Ag/AgCl and gold electrodes depends on the stability requirements, environmental conditions, and the specific electrochemical or analytical techniques involved in the application.
Silver-Silver Chloride vs Gold Electrode Infographic
 electrown.com
electrown.com