Conductivity sensors measure the ability of a solution to conduct electrical current, providing critical data on ion concentration and purity, while pH sensors determine the acidity or alkalinity of a solution, essential for monitoring chemical reactions and environmental conditions. To understand which sensor suits Your specific needs and applications, explore the detailed comparison in the rest of this article.
Table of Comparison
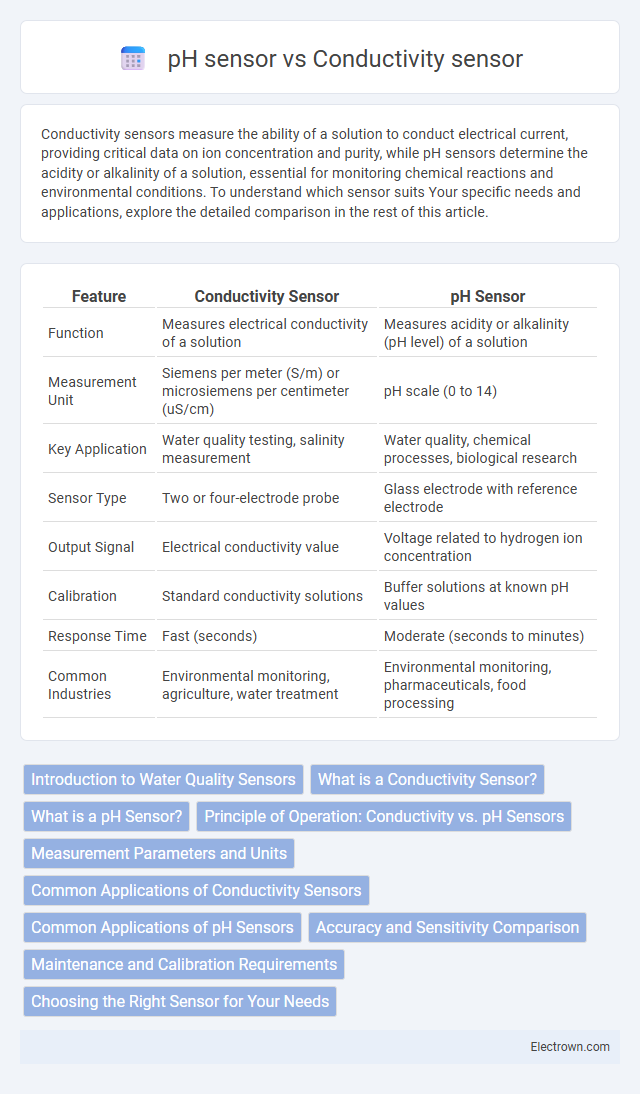
| Feature | Conductivity Sensor | pH Sensor |
|---|---|---|
| Function | Measures electrical conductivity of a solution | Measures acidity or alkalinity (pH level) of a solution |
| Measurement Unit | Siemens per meter (S/m) or microsiemens per centimeter (uS/cm) | pH scale (0 to 14) |
| Key Application | Water quality testing, salinity measurement | Water quality, chemical processes, biological research |
| Sensor Type | Two or four-electrode probe | Glass electrode with reference electrode |
| Output Signal | Electrical conductivity value | Voltage related to hydrogen ion concentration |
| Calibration | Standard conductivity solutions | Buffer solutions at known pH values |
| Response Time | Fast (seconds) | Moderate (seconds to minutes) |
| Common Industries | Environmental monitoring, agriculture, water treatment | Environmental monitoring, pharmaceuticals, food processing |
Introduction to Water Quality Sensors
Water quality sensors such as conductivity sensors and pH sensors play crucial roles in monitoring the chemical properties of water. Conductivity sensors measure the ability of water to conduct electrical current, providing data on ion concentration and overall water purity. pH sensors detect the hydrogen ion concentration, indicating the acidity or alkalinity of the water, which directly affects aquatic life and water treatment processes.
What is a Conductivity Sensor?
A conductivity sensor measures the ability of a solution to conduct electrical current, providing critical data on the ionic content and concentration in liquids. Unlike a pH sensor that gauges the hydrogen ion activity to determine acidity or alkalinity, conductivity sensors quantify total dissolved solids, salts, and impurities. Your choice between these sensors depends on whether you need to monitor the electrolyte concentration (conductivity) or the solution's pH level for accurate water quality analysis.
What is a pH Sensor?
A pH sensor measures the hydrogen ion concentration in a solution, providing a precise indication of its acidity or alkalinity on a scale from 0 to 14. It typically consists of a glass electrode and a reference electrode that generate a voltage proportional to the pH level. Unlike conductivity sensors that measure the ionic conductivity of a solution, pH sensors specifically detect the activity of hydrogen ions, making them essential for applications in water quality monitoring, agriculture, and chemical processing.
Principle of Operation: Conductivity vs. pH Sensors
Conductivity sensors measure the ability of a solution to conduct electrical current, relying on the presence of ions in the liquid that carry charge between electrodes. pH sensors operate based on the measurement of hydrogen ion activity in a solution, typically using a glass electrode that generates a voltage corresponding to the pH level. While conductivity sensors quantify ionic concentration, pH sensors determine the acidity or alkalinity by detecting hydrogen ion concentration.
Measurement Parameters and Units
Conductivity sensors measure the ability of a solution to conduct electrical current, with results expressed in microsiemens per centimeter (uS/cm) or millisiemens per centimeter (mS/cm). pH sensors quantify the hydrogen ion concentration to determine acidity or alkalinity, providing readings on a scale from 0 to 14 pH units. Understanding the distinct measurement parameters helps you select the appropriate sensor for accurate monitoring of water quality or chemical processes.
Common Applications of Conductivity Sensors
Conductivity sensors are widely used in water quality monitoring, industrial process control, and environmental testing due to their ability to measure the ionic content of a solution. These sensors provide critical data in applications like wastewater treatment, food and beverage production, and chemical manufacturing to ensure consistent product quality and regulatory compliance. Your choice between a conductivity sensor and a pH sensor depends on whether you need to measure the electrical conductivity related to ion concentration or the hydrogen ion activity indicating acidity.
Common Applications of pH Sensors
pH sensors are commonly used in water quality monitoring, agriculture, aquariums, and chemical processing to measure the acidity or alkalinity of solutions accurately. These sensors are essential for ensuring optimal conditions in hydroponics, wastewater treatment, and pharmaceutical manufacturing. Your choice of a pH sensor can significantly impact process control and environmental compliance due to their precise detection of hydrogen ion concentration.
Accuracy and Sensitivity Comparison
Conductivity sensors provide high accuracy in measuring ionic concentration by detecting electrical current flow, making them highly sensitive to dissolved salts and impurities. pH sensors, on the other hand, measure hydrogen ion activity with precision, offering sensitivity to subtle changes in acidity or alkalinity. When selecting between the two, your choice depends on whether you need detailed analysis of ionic content or precise monitoring of pH levels, as both sensors excel in accuracy but target different chemical properties.
Maintenance and Calibration Requirements
Conductivity sensors require regular cleaning to prevent electrode fouling and ensure accurate measurements, with calibration typically performed using standard conductivity solutions. pH sensors demand frequent maintenance including electrode conditioning, cleaning, and storage in appropriate solutions to avoid drift and response degradation, with calibration carried out using buffer solutions at multiple pH points. Both sensor types benefit from routine calibration and maintenance schedules to maintain precision and extend operational lifespan in industrial or laboratory settings.
Choosing the Right Sensor for Your Needs
Choosing the right sensor depends on the specific water quality parameters you need to measure: conductivity sensors detect the ability of water to conduct electrical current, indicating ion concentration, while pH sensors measure the acidity or alkalinity of a solution. Conductivity sensors are ideal for monitoring salinity, total dissolved solids (TDS), and overall ionic content, making them essential in water treatment and industrial processes. pH sensors provide critical data for chemical balance, environmental monitoring, and biological applications where maintaining proper acidity is crucial.
Conductivity sensor vs pH sensor Infographic
 electrown.com
electrown.com