MRI-compatible medical devices are specifically designed to function safely within the strong magnetic fields of MRI machines, minimizing risks such as heating, displacement, or interference with imaging quality. Understanding the differences between these and standard medical devices can help you make informed decisions about patient safety and diagnostic accuracy; explore the rest of the article to learn more.
Table of Comparison
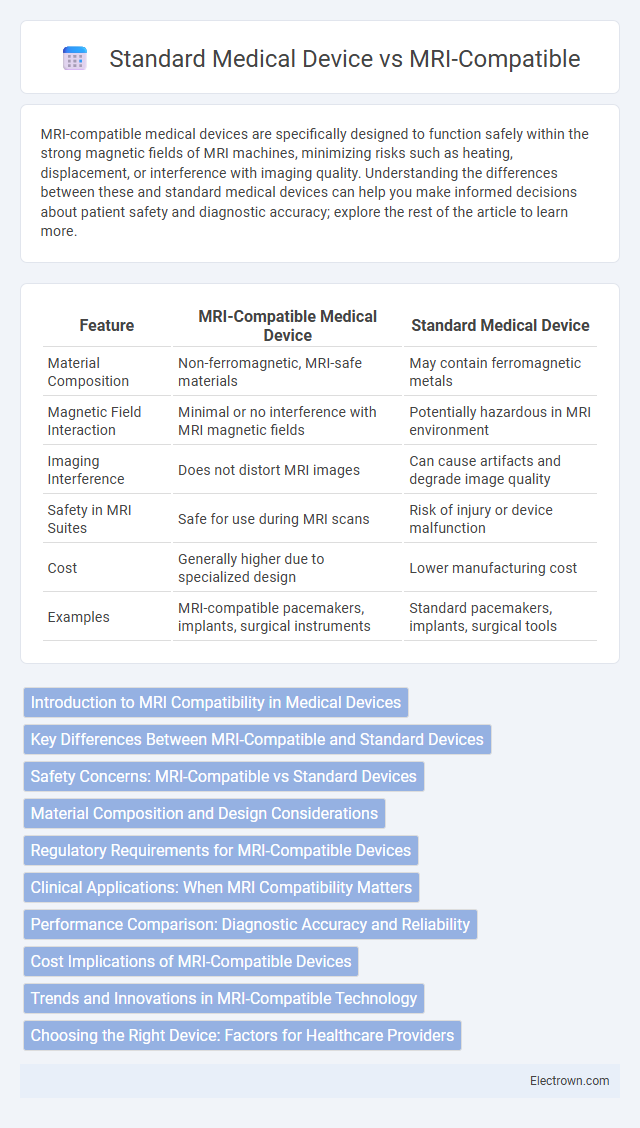
| Feature | MRI-Compatible Medical Device | Standard Medical Device |
|---|---|---|
| Material Composition | Non-ferromagnetic, MRI-safe materials | May contain ferromagnetic metals |
| Magnetic Field Interaction | Minimal or no interference with MRI magnetic fields | Potentially hazardous in MRI environment |
| Imaging Interference | Does not distort MRI images | Can cause artifacts and degrade image quality |
| Safety in MRI Suites | Safe for use during MRI scans | Risk of injury or device malfunction |
| Cost | Generally higher due to specialized design | Lower manufacturing cost |
| Examples | MRI-compatible pacemakers, implants, surgical instruments | Standard pacemakers, implants, surgical tools |
Introduction to MRI Compatibility in Medical Devices
MRI-compatible medical devices are specifically designed to function safely and effectively within the strong magnetic fields of MRI scanners, minimizing risks such as device malfunction or patient injury. Standard medical devices, not engineered for MRI environments, may contain ferromagnetic materials or electronics that can interfere with imaging or pose safety hazards during scanning. Ensuring MRI compatibility involves rigorous testing for materials, device functionality, and electromagnetic interference to maintain accurate diagnostics and patient safety.
Key Differences Between MRI-Compatible and Standard Devices
MRI-compatible medical devices are specifically designed with non-ferromagnetic materials to ensure safety and functionality within the strong magnetic fields generated by MRI machines. Standard medical devices often contain metals that can interfere with MRI imaging and pose safety risks, such as device displacement or malfunction. You should choose MRI-compatible devices to guarantee accurate diagnostics and patient safety during MRI procedures.
Safety Concerns: MRI-Compatible vs Standard Devices
MRI-compatible medical devices are specifically designed with non-ferromagnetic materials, reducing the risk of projectile hazards, heating, or malfunction during an MRI scan. Standard medical devices may pose safety concerns in the MRI environment, including interference with imaging quality and potential injury due to magnetic forces. Ensuring your device is MRI-compatible is crucial to protect patient safety and maintain diagnostic accuracy during MRI procedures.
Material Composition and Design Considerations
MRI-compatible medical devices are constructed from non-ferromagnetic materials such as titanium, plastics, and ceramics to prevent magnetic interference and ensure patient safety during MRI scans. Design considerations emphasize minimizing metallic content, avoiding conductive loops, and ensuring device functionality without image distortion or heating effects. Standard medical devices often use stainless steel or other ferromagnetic metals, which can cause artifacts, device malfunction, or pose safety risks in high magnetic fields.
Regulatory Requirements for MRI-Compatible Devices
MRI-compatible devices must comply with stringent regulatory requirements developed by agencies such as the FDA and ISO, ensuring safety and compatibility within magnetic resonance imaging environments. These devices undergo rigorous testing for electromagnetic interference, material non-magnetism, and safe function during MRI scans to prevent patient harm and image distortion. Your selection of MRI-compatible equipment should prioritize certified compliance with ASTM standards F2503 and EN 60601-2-33 for optimal safety and effectiveness.
Clinical Applications: When MRI Compatibility Matters
MRI-compatible medical devices are essential in clinical applications involving magnetic resonance imaging to ensure patient safety and accurate diagnostics. Unlike standard medical devices that can malfunction or pose risks in the strong magnetic field of an MRI scanner, MRI-compatible devices are designed with non-ferromagnetic materials and specialized components to prevent interference. Your choice of MRI-compatible equipment is critical when conducting scans for neurological, musculoskeletal, or cardiovascular conditions that require real-time imaging without compromising device functionality or patient well-being.
Performance Comparison: Diagnostic Accuracy and Reliability
MRI-compatible medical devices maintain high diagnostic accuracy and reliability by minimizing interference with magnetic fields, ensuring precise imaging results during MRI procedures. Standard medical devices may pose risks of artifacts or signal distortion, potentially compromising diagnostic clarity and leading to less reliable outcomes. Choosing MRI-compatible devices enhances your ability to obtain accurate diagnostic information without sacrificing device performance or patient safety.
Cost Implications of MRI-Compatible Devices
MRI-compatible devices generally incur higher initial costs due to specialized materials and engineering required to prevent interference with magnetic fields. Maintenance and replacement expenses also tend to be greater, reflecting rigorous safety standards and limited manufacturer options. Healthcare providers must weigh these cost implications against patient safety, improved diagnostic accuracy, and potential reductions in procedural complications.
Trends and Innovations in MRI-Compatible Technology
MRI-compatible medical devices are rapidly evolving with innovations such as non-metallic materials, wireless technology, and advanced shielding techniques to ensure safety and functionality within high magnetic fields. Trends highlight a growing integration of smart sensors and remote monitoring capabilities, enhancing diagnostic accuracy and patient comfort during MRI procedures. Your choice of MRI-compatible technology can significantly improve procedural efficiency and reduce the risk of device interference or patient injury.
Choosing the Right Device: Factors for Healthcare Providers
Healthcare providers must consider device safety, compatibility with MRI environments, and patient conditions when choosing between MRI-compatible and standard medical devices. MRI-compatible devices minimize risks of malfunction or injury during imaging, offering non-magnetic materials and specialized designs for strong magnetic fields. Cost, device availability, and diagnostic requirements further influence the selection to ensure optimal patient care and imaging accuracy.
MRI-Compatible vs Standard Medical Device Infographic
 electrown.com
electrown.com