Electroluminescence occurs when a material emits light in response to an electric current or field, while photoluminescence involves light emission after the material absorbs photons. Understanding the differences in mechanisms and applications can enhance your grasp of advanced lighting and display technologies; read on to explore the detailed distinctions and uses.
Table of Comparison
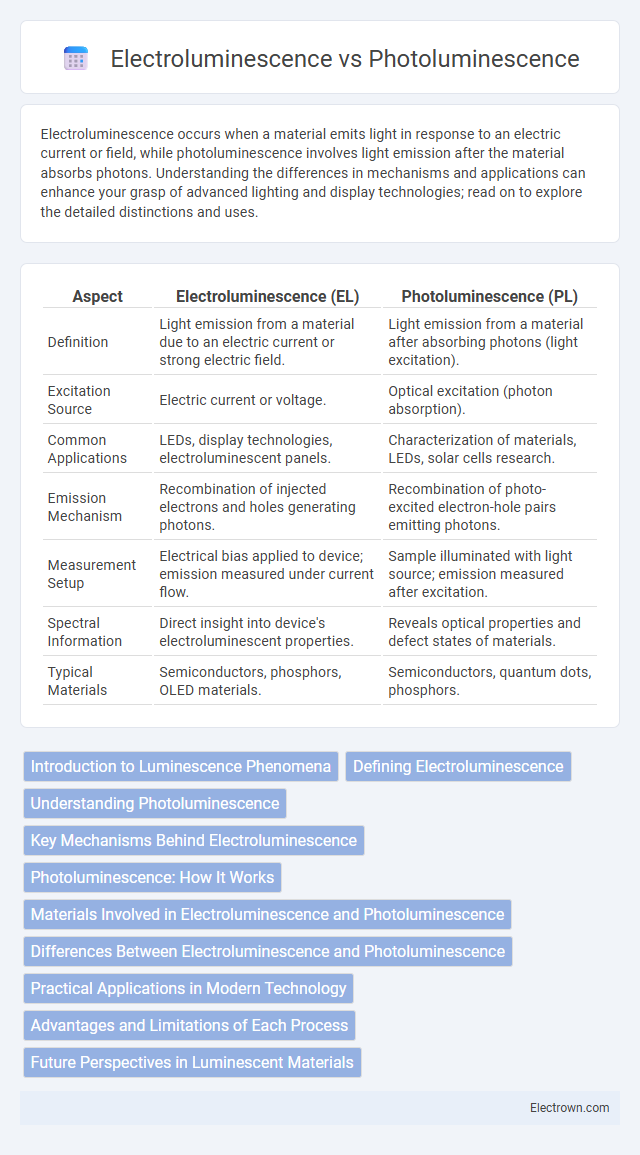
| Aspect | Electroluminescence (EL) | Photoluminescence (PL) |
|---|---|---|
| Definition | Light emission from a material due to an electric current or strong electric field. | Light emission from a material after absorbing photons (light excitation). |
| Excitation Source | Electric current or voltage. | Optical excitation (photon absorption). |
| Common Applications | LEDs, display technologies, electroluminescent panels. | Characterization of materials, LEDs, solar cells research. |
| Emission Mechanism | Recombination of injected electrons and holes generating photons. | Recombination of photo-excited electron-hole pairs emitting photons. |
| Measurement Setup | Electrical bias applied to device; emission measured under current flow. | Sample illuminated with light source; emission measured after excitation. |
| Spectral Information | Direct insight into device's electroluminescent properties. | Reveals optical properties and defect states of materials. |
| Typical Materials | Semiconductors, phosphors, OLED materials. | Semiconductors, quantum dots, phosphors. |
Introduction to Luminescence Phenomena
Luminescence phenomena encompass processes where materials emit light through electronic transitions without heat generation, including electroluminescence and photoluminescence. Electroluminescence involves light emission triggered by an electric field or current, commonly found in LEDs and display technologies. Photoluminescence occurs when materials absorb photons and re-emit light, integral in applications such as fluorescence spectroscopy and phosphor materials.
Defining Electroluminescence
Electroluminescence is the phenomenon where materials emit light in response to an electric current or a strong electric field, distinguishing it from photoluminescence, which relies on light absorption. This effect occurs when electrons recombine with holes within a semiconductor or insulating material, releasing energy as photons. Your understanding of electroluminescence is essential for applications such as OLED displays and light-emitting diodes (LEDs).
Understanding Photoluminescence
Photoluminescence is the emission of light from a material after it absorbs photons, a process primarily used to analyze the electronic and structural properties of semiconductors and nanomaterials. In photoluminescence, your material's atoms or molecules absorb incoming light, exciting electrons to higher energy states, which then release energy as they return to their ground state, emitting visible or near-visible light. This optical phenomenon is key for applications in LED technology, solar cells, and fluorescence microscopy, providing insights that differ substantially from electroluminescence, where light emission is driven by an electric current.
Key Mechanisms Behind Electroluminescence
Electroluminescence occurs when an electric current or a strong electric field passes through a material, causing electrons to recombine with holes and emit photons. This process is primarily driven by the injection of charge carriers from electrodes into a semiconductor or organic material, where radiative recombination takes place. Unlike photoluminescence, which relies on the absorption of photons to excite electrons, electroluminescence is powered by electrical energy directly converting to light emission.
Photoluminescence: How It Works
Photoluminescence occurs when a material absorbs photons and then re-emits them as light, a process driven by the excitation of electrons to higher energy states followed by their return to ground state, releasing energy as visible light. This phenomenon is widely used in applications like fluorescence imaging, light-emitting diodes (LEDs), and solar cell technologies to analyze material properties and improve device performance. Understanding photoluminescence helps you optimize materials for better light emission efficiency and spectral characteristics.
Materials Involved in Electroluminescence and Photoluminescence
Electroluminescence typically involves semiconductor materials such as gallium arsenide (GaAs), gallium nitride (GaN), and organic compounds used in OLEDs, where charge injection induces light emission. Photoluminescence relies on materials like quantum dots, rare-earth-doped crystals, and fluorescent dyes that emit light upon photon excitation. Both phenomena exploit different mechanisms in materials, with electroluminescence requiring electrical energy and photoluminescence driven by optical excitation.
Differences Between Electroluminescence and Photoluminescence
Electroluminescence occurs when a material emits light in response to an electric current or electric field, whereas photoluminescence is triggered by the absorption of photons from an external light source. In electroluminescence, charge carriers such as electrons and holes recombine to produce light, while photoluminescence relies on excited electrons returning to their ground state and releasing photons. The governing mechanisms and excitation methods distinguish electroluminescence in devices like LEDs from photoluminescence observed in fluorescence and phosphorescence phenomena.
Practical Applications in Modern Technology
Electroluminescence powers devices like OLED screens and LED lighting, converting electrical energy into visible light with high efficiency and color precision. Photoluminescence is critical in applications such as fluorescence-based sensors, bioimaging, and solar cells, where materials absorb photons and re-emit them for detection or energy conversion. Understanding these mechanisms helps you choose the right technology for developing advanced display systems or optical sensors in modern electronics.
Advantages and Limitations of Each Process
Electroluminescence offers the advantage of direct electrical excitation, enabling efficient, compact light sources such as OLED displays with low power consumption. Photoluminescence, relying on optical excitation, provides high sensitivity for applications like fluorescence microscopy, but requires an external light source, limiting portability. Your choice depends on whether electrical efficiency or precise optical detection is prioritized.
Future Perspectives in Luminescent Materials
Emerging research in electroluminescence (EL) and photoluminescence (PL) materials highlights advancements in perovskite and organic light-emitting diodes (OLEDs) for high-efficiency, low-energy luminescent devices. Future perspectives emphasize enhancing stability, color purity, and tunability through nanomaterials and quantum dots to revolutionize display technologies and flexible electronics. Integration of these luminescent materials with scalable fabrication techniques promises transformative applications in wearable devices, lighting, and bio-imaging.
Electroluminescence vs Photoluminescence Infographic
 electrown.com
electrown.com