Isothermal operation maintains a constant temperature throughout the process by allowing heat exchange with the surroundings, while adiabatic operation prevents any heat transfer, causing temperature to change due to work done on or by the system. Understanding these differences can help you optimize your thermal system's performance; explore the rest of the article to discover how each method impacts efficiency and design choices.
Table of Comparison
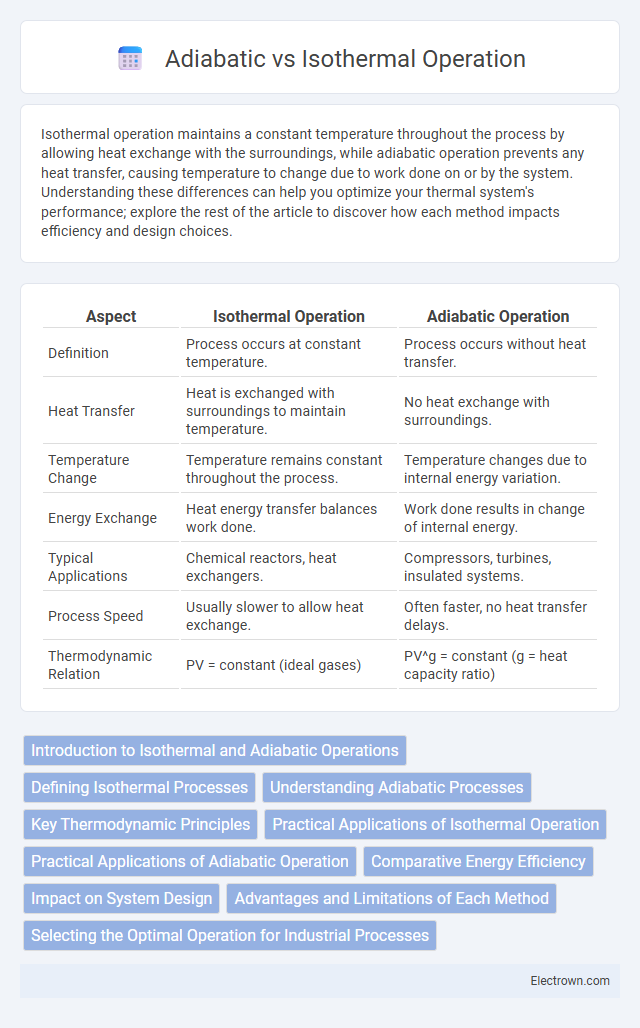
| Aspect | Isothermal Operation | Adiabatic Operation |
|---|---|---|
| Definition | Process occurs at constant temperature. | Process occurs without heat transfer. |
| Heat Transfer | Heat is exchanged with surroundings to maintain temperature. | No heat exchange with surroundings. |
| Temperature Change | Temperature remains constant throughout the process. | Temperature changes due to internal energy variation. |
| Energy Exchange | Heat energy transfer balances work done. | Work done results in change of internal energy. |
| Typical Applications | Chemical reactors, heat exchangers. | Compressors, turbines, insulated systems. |
| Process Speed | Usually slower to allow heat exchange. | Often faster, no heat transfer delays. |
| Thermodynamic Relation | PV = constant (ideal gases) | PV^g = constant (g = heat capacity ratio) |
Introduction to Isothermal and Adiabatic Operations
Isothermal operation involves maintaining a constant temperature throughout the process, ensuring heat exchange with the surroundings to counteract any temperature changes. Adiabatic operation occurs without heat transfer, so the system's temperature changes as a result of work done on or by the system. These distinct approaches impact energy efficiency and are critical in designing thermodynamic cycles and industrial processes.
Defining Isothermal Processes
Isothermal processes occur at a constant temperature, meaning the system's internal energy remains unchanged as heat exchange with the surroundings compensates for work done. These processes are characterized by the equation PV = constant, where pressure and volume inversely vary to maintain equilibrium. Isothermal operation is crucial in applications like gas compression and expansion where temperature control preserves material integrity.
Understanding Adiabatic Processes
Adiabatic processes occur without heat exchange between a system and its surroundings, causing changes in temperature and pressure solely from internal energy shifts. In an adiabatic operation, the system's entropy remains constant, and energy transfer happens exclusively as work, making it crucial in thermodynamic cycles like compressors and turbines. Understanding these processes helps optimize your system's efficiency by controlling temperature changes during rapid gas expansions or compressions.
Key Thermodynamic Principles
Isothermal operation maintains a constant temperature by allowing heat exchange with the surroundings, ensuring the internal energy remains unchanged during the process. Adiabatic operation prohibits heat transfer, causing temperature changes as work is done on or by the system, directly affecting its internal energy. Understanding these key thermodynamic principles helps you optimize energy efficiency and process design in systems like compressors, turbines, and heat exchangers.
Practical Applications of Isothermal Operation
Isothermal operation is widely used in processes where temperature control is crucial, such as in chemical reactors for polymerization and fermentation where consistent heat levels ensure product quality. Maintaining constant temperature during gas compression in refrigeration cycles enhances efficiency and prevents equipment damage. Your systems benefit from isothermal conditions when precise thermal management improves stability and performance in sensitive industrial applications.
Practical Applications of Adiabatic Operation
Adiabatic operation is widely utilized in industrial gas compression and expansion processes, where minimizing heat exchange enhances energy efficiency. It is crucial in gas turbines and compressors to achieve rapid process cycles without heat loss, preserving the internal energy. Practical applications also include cryogenic processes and certain chemical reactors where maintaining temperature integrity is essential for reaction control.
Comparative Energy Efficiency
Isothermal operation maintains constant temperature by allowing heat exchange with the surroundings, resulting in lower work input and higher energy efficiency for processes such as compression or expansion. Adiabatic operation involves no heat transfer, causing temperature changes that typically increase work requirements and reduce overall efficiency. Your choice between these modes directly impacts energy consumption, with isothermal processes generally offering superior energy efficiency in practical applications.
Impact on System Design
Isothermal operation requires efficient heat exchange systems to maintain constant temperature, influencing material selection and thermal management design. Adiabatic operation, by contrast, eliminates heat transfer, necessitating insulation and often leading to higher pressure and temperature variations within the system. Your choice between these modes significantly impacts equipment sizing, safety considerations, and energy consumption in the overall system design.
Advantages and Limitations of Each Method
Isothermal operation maintains constant temperature, enhancing energy efficiency and reducing thermal stresses but requires effective heat exchange systems, which can increase complexity and cost. Adiabatic operation involves no heat transfer, simplifying equipment design and lowering operational costs but often leads to temperature fluctuations that may affect product quality or reaction rates. Selecting between methods depends on process requirements, with isothermal favoring temperature-sensitive reactions and adiabatic suited for less temperature-dependent operations.
Selecting the Optimal Operation for Industrial Processes
Isothermal operation maintains a constant temperature by allowing heat exchange with the surroundings, ideal for processes requiring precise thermal control and energy efficiency, such as in chemical reactors or fermentation. Adiabatic operation involves no heat transfer, resulting in temperature changes due to compression or expansion, suitable for rapid processes like gas turbines or in situations where insulation is more practical than cooling or heating. Selecting the optimal operation depends on factors such as process energy requirements, thermal sensitivity, system complexity, and cost considerations to maximize efficiency and product quality in industrial applications.
Isothermal vs Adiabatic operation Infographic
 electrown.com
electrown.com