N-type semiconductors have extra electrons as charge carriers, while p-type semiconductors contain holes that act as positive charge carriers, each essential for creating electronic components like diodes and transistors. Explore the differences and applications of n-type vs p-type materials to understand how they impact your electronic devices.
Table of Comparison
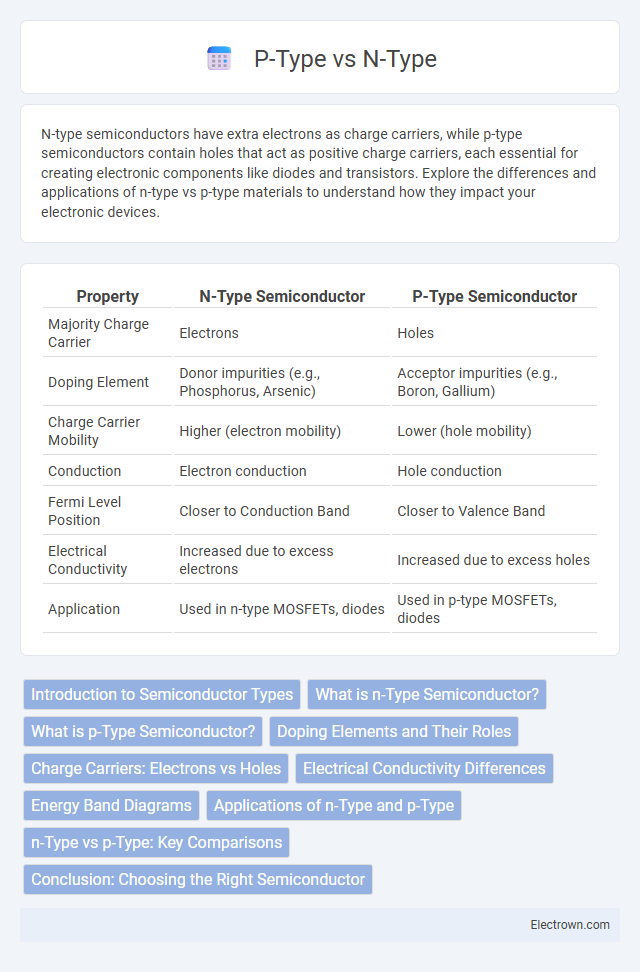
| Property | N-Type Semiconductor | P-Type Semiconductor |
|---|---|---|
| Majority Charge Carrier | Electrons | Holes |
| Doping Element | Donor impurities (e.g., Phosphorus, Arsenic) | Acceptor impurities (e.g., Boron, Gallium) |
| Charge Carrier Mobility | Higher (electron mobility) | Lower (hole mobility) |
| Conduction | Electron conduction | Hole conduction |
| Fermi Level Position | Closer to Conduction Band | Closer to Valence Band |
| Electrical Conductivity | Increased due to excess electrons | Increased due to excess holes |
| Application | Used in n-type MOSFETs, diodes | Used in p-type MOSFETs, diodes |
Introduction to Semiconductor Types
N-type and P-type semiconductors are fundamental materials in electronics, distinguished by their charge carriers: electrons for N-type and holes for P-type. In N-type semiconductors, donor impurities add extra electrons, enhancing electrical conductivity, while P-type materials gain holes through acceptor impurities, creating positive charge carriers. Your understanding of these semiconductor types is essential for grasping the operation of devices like diodes and transistors.
What is n-Type Semiconductor?
N-type semiconductor is a material doped with elements that have more valence electrons than the base semiconductor, typically silicon. This extra electron acts as the majority charge carrier, enhancing the material's electrical conductivity. Your electronic devices rely on n-type semiconductors for efficient electron flow in components like transistors and diodes.
What is p-Type Semiconductor?
A p-type semiconductor is a material doped with elements that create an abundance of holes, or positive charge carriers, within its crystal lattice. These holes facilitate electrical conductivity by allowing electrons to move and fill the vacancies, effectively making the p-type region positively charged. Understanding your p-type semiconductor is crucial for designing devices like diodes and transistors where controlling charge flow is essential.
Doping Elements and Their Roles
N-type semiconductors are doped with elements such as phosphorus or arsenic, which have five valence electrons, providing extra electrons that enhance electrical conductivity. P-type semiconductors use doping elements like boron or gallium, possessing three valence electrons, creating "holes" that act as positive charge carriers. Your choice between n-type and p-type doping depends on the desired conductivity and device functionality in electronic applications.
Charge Carriers: Electrons vs Holes
N-type semiconductors have electrons as the majority charge carriers, introduced by doping with elements that have more valence electrons than the host material, enhancing electrical conductivity through increased electron availability. P-type semiconductors rely on holes as the majority charge carriers, created by doping with elements that have fewer valence electrons, resulting in positive charge carriers that facilitate current flow by the movement of these vacancies. Understanding the distinction between electrons in n-type and holes in p-type is crucial for optimizing the performance of semiconductor devices in your electronic applications.
Electrical Conductivity Differences
N-type semiconductors exhibit higher electrical conductivity due to the abundance of free electrons, which act as the majority charge carriers. In contrast, p-type semiconductors rely on holes as majority carriers, resulting in generally lower conductivity because hole mobility is typically less than that of electrons. Understanding these conductivity differences is crucial for optimizing Your electronic device performance.
Energy Band Diagrams
N-type semiconductors have an energy band diagram where donor energy levels lie just below the conduction band, resulting in excess electrons that increase conductivity. P-type semiconductors feature acceptor energy levels just above the valence band, creating holes that facilitate positive charge carrier movement. The difference in these band structures directly impacts charge carrier concentration and electrical properties in semiconductor devices.
Applications of n-Type and p-Type
N-type semiconductors are extensively used in electronic devices such as transistors, diodes, and integrated circuits due to their high electron mobility, making them essential for efficient conduction. P-type semiconductors are crucial in creating p-n junctions, which are the foundation of photovoltaic cells, light-emitting diodes (LEDs), and various sensors. Your choice between n-type and p-type materials directly influences the performance and functionality of semiconductor-based applications.
n-Type vs p-Type: Key Comparisons
N-type semiconductors are doped with elements that have extra electrons, resulting in negatively charged carriers, while p-type semiconductors contain dopants that create holes, or positively charged carriers. The electrical conductivity in n-type materials is primarily driven by electron flow, whereas in p-type materials, it is facilitated by hole movement. Understanding these key differences influences your choice in designing electronic components such as transistors and diodes.
Conclusion: Choosing the Right Semiconductor
Selecting the appropriate semiconductor type depends on the specific application requirements, where n-type materials provide higher electron mobility and better conductivity, making them ideal for high-speed devices. P-type semiconductors, with their hole conduction properties, are often preferred in complementary metal-oxide-semiconductor (CMOS) technology for balanced electrical performance. Understanding the electrical characteristics and doping effects ensures optimal device efficiency and functionality.
n-type vs p-type Infographic
 electrown.com
electrown.com