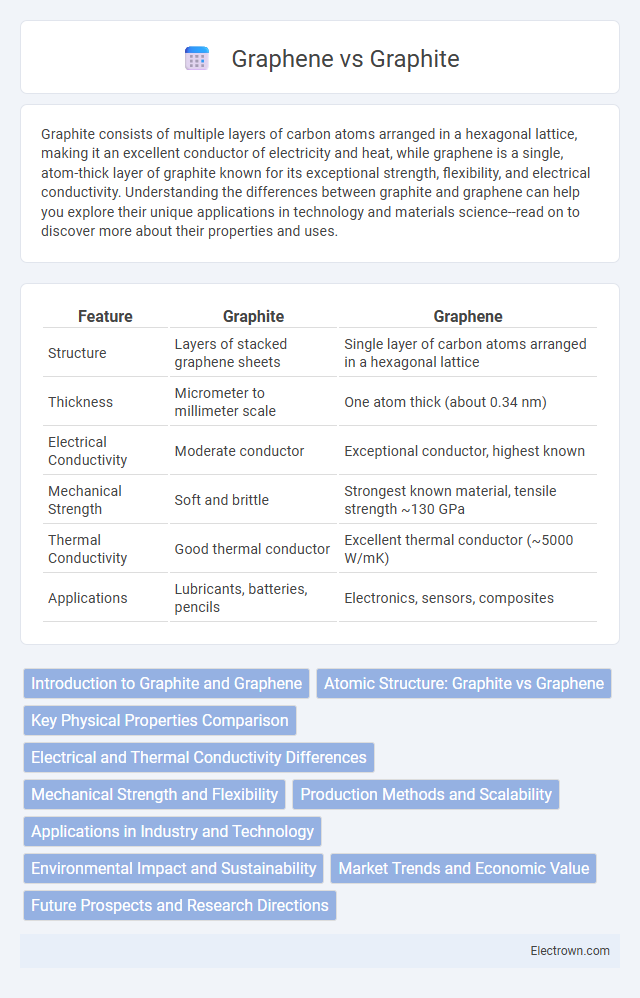
Graphite consists of multiple layers of carbon atoms arranged in a hexagonal lattice, making it an excellent conductor of electricity and heat, while graphene is a single, atom-thick layer of graphite known for its exceptional strength, flexibility, and electrical conductivity. Understanding the differences between graphite and graphene can help you explore their unique applications in technology and materials science--read on to discover more about their properties and uses.
Table of Comparison
| Feature | Graphite | Graphene |
|---|---|---|
| Structure | Layers of stacked graphene sheets | Single layer of carbon atoms arranged in a hexagonal lattice |
| Thickness | Micrometer to millimeter scale | One atom thick (about 0.34 nm) |
| Electrical Conductivity | Moderate conductor | Exceptional conductor, highest known |
| Mechanical Strength | Soft and brittle | Strongest known material, tensile strength ~130 GPa |
| Thermal Conductivity | Good thermal conductor | Excellent thermal conductor (~5000 W/mK) |
| Applications | Lubricants, batteries, pencils | Electronics, sensors, composites |
Introduction to Graphite and Graphene
Graphite is a naturally occurring form of carbon composed of multiple layers of carbon atoms arranged in a hexagonal lattice, providing excellent conductivity and lubricating properties. Graphene is a single layer of carbon atoms extracted from graphite, renowned for its extraordinary strength, flexibility, and superior electrical and thermal conductivity. The atomic thickness and unique electronic characteristics of graphene distinguish it from bulk graphite, enabling advanced applications in electronics and materials science.
Atomic Structure: Graphite vs Graphene
Graphite consists of multiple layers of carbon atoms arranged in a hexagonal lattice, with weak van der Waals forces holding these layers together, allowing easy exfoliation into thinner sheets. Graphene is a single atomic layer of carbon atoms tightly packed in a two-dimensional honeycomb lattice, exhibiting exceptional mechanical strength and electrical conductivity due to its sp2 hybridized carbon atoms. The atomic structure of graphene provides superior electron mobility compared to the multilayer stacking in graphite, making it a unique material for advanced technological applications.
Key Physical Properties Comparison
Graphite consists of multiple layers of carbon atoms arranged in a hexagonal lattice, providing it with excellent electrical conductivity and lubrication properties due to weak interlayer forces. Graphene, a single layer of carbon atoms in the same hexagonal structure, exhibits superior mechanical strength, exceptional electrical conductivity, and remarkable thermal conductivity. Your choice between graphite and graphene depends on the required physical properties, such as flexibility, conductivity, and strength, for specific applications.
Electrical and Thermal Conductivity Differences
Graphene exhibits exceptional electrical conductivity, with electron mobility surpassing 200,000 cm2/V*s, far exceeding graphite's conductivity due to its two-dimensional structure and delocalized p-electrons. Thermal conductivity in graphene reaches up to 5300 W/m*K, significantly higher than graphite's typical range of 100-500 W/m*K, making graphene superior for heat dissipation in electronic applications. These differences arise from graphene's single-atom thickness and high crystallinity, which reduce phonon scattering compared to the multi-layered structure of graphite.
Mechanical Strength and Flexibility
Graphene exhibits exceptional mechanical strength with a tensile strength of approximately 130 gigapascals, making it one of the strongest known materials, while graphite, composed of many graphene layers, is much weaker due to interlayer sliding. The flexibility of graphene is also superior, allowing it to bend without breaking to great degrees, unlike the brittle nature of bulk graphite. This combination of high tensile strength and remarkable flexibility gives graphene distinct advantages in advanced materials and flexible electronics.
Production Methods and Scalability
Graphite is commonly produced through mining and mechanical exfoliation, offering large-scale availability but limited purity for advanced applications. Graphene production involves chemical vapor deposition (CVD), liquid-phase exfoliation, or epitaxial growth, enabling higher quality and functionality but facing challenges in scalability and cost-efficiency. Your choice depends on balancing graphene's superior performance with graphite's well-established, scalable production infrastructure.
Applications in Industry and Technology
Graphite's layered structure makes it ideal for lubrication, batteries, and refractories, while graphene's single-atom thickness and exceptional conductivity revolutionize electronics, sensors, and energy storage. Industrial applications of graphite include electrode manufacturing in steel production and heat dissipation in nuclear reactors, whereas graphene enhances flexible displays, high-frequency transistors, and supercapacitors. The contrasting properties of graphite and graphene enable diverse technological advancements across energy, materials science, and nanotechnology sectors.
Environmental Impact and Sustainability
Graphite mining involves significant environmental disruption, including habitat destruction and water pollution, while graphene production, although currently energy-intensive, offers prospects for sustainable methods through advances in green chemistry and scalable synthesis techniques. Graphene's potential to enhance energy storage, lightweight materials, and water purification supports sustainable development goals by reducing carbon footprints and resource consumption. Transitioning from traditional graphite use to graphene-based technologies could significantly mitigate environmental impact through improved efficiency and recyclability.
Market Trends and Economic Value
Graphene commands a rapidly growing market driven by its superior electrical conductivity, mechanical strength, and versatility in applications ranging from electronics to energy storage, projecting a CAGR of over 40% through 2030. Graphite remains a cornerstone commodity with stable demand primarily from battery anode production, especially in lithium-ion batteries, valued at approximately $13 billion globally in 2023. Investment shifts increasingly favor graphene due to its higher economic value per unit weight and expanding integration in advanced technologies, signaling a strategic transition in the materials market.
Future Prospects and Research Directions
Graphene's exceptional electrical conductivity, mechanical strength, and thermal properties position it as a transformative material in future tech innovations, surpassing graphite's traditional uses. Research is advancing towards scalable production methods and integration into electronics, energy storage, and composite materials to unlock graphene's full potential. Your understanding of these developments can guide strategic investments and research initiatives in next-generation nanomaterials.
graphite vs graphene Infographic
 electrown.com
electrown.com