Surface energy refers to the excess energy at the surface of a material compared to its bulk, influencing how liquids spread or adhere to that surface, while surface tension is the cohesive force at the liquid's surface that causes it to minimize area and resist external force. Understanding these concepts helps you better grasp adhesion mechanisms in applications like coatings, adhesives, and material compatibility; continue reading to explore their differences and real-world implications.
Table of Comparison
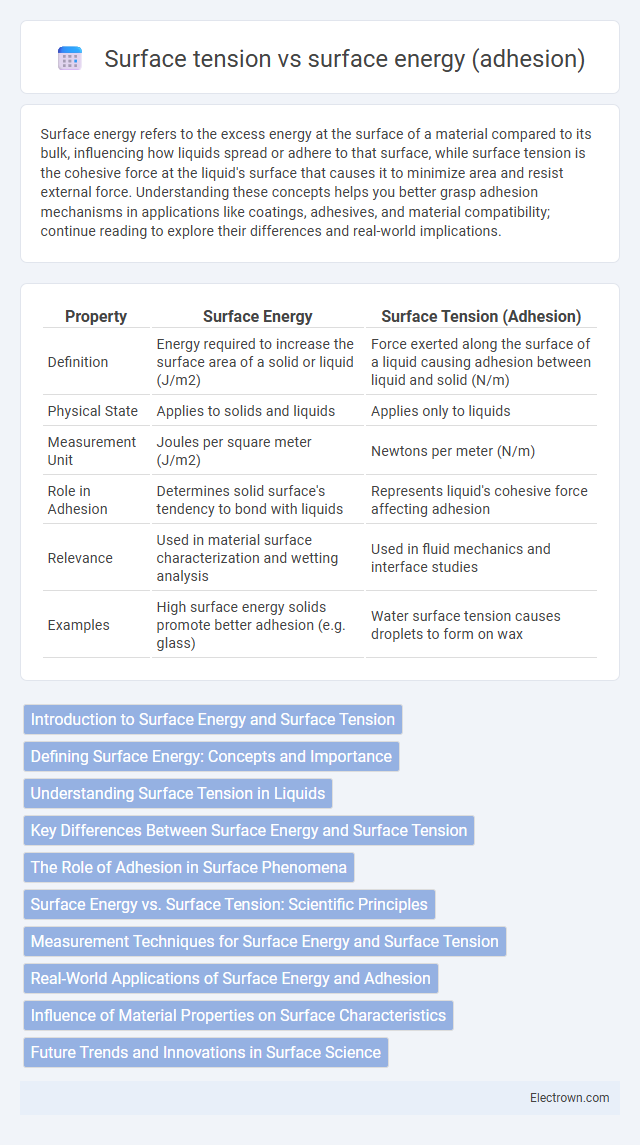
| Property | Surface Energy | Surface Tension (Adhesion) |
|---|---|---|
| Definition | Energy required to increase the surface area of a solid or liquid (J/m2) | Force exerted along the surface of a liquid causing adhesion between liquid and solid (N/m) |
| Physical State | Applies to solids and liquids | Applies only to liquids |
| Measurement Unit | Joules per square meter (J/m2) | Newtons per meter (N/m) |
| Role in Adhesion | Determines solid surface's tendency to bond with liquids | Represents liquid's cohesive force affecting adhesion |
| Relevance | Used in material surface characterization and wetting analysis | Used in fluid mechanics and interface studies |
| Examples | High surface energy solids promote better adhesion (e.g. glass) | Water surface tension causes droplets to form on wax |
Introduction to Surface Energy and Surface Tension
Surface energy refers to the excess energy at the surface of a material compared to its bulk, driving phenomena like adhesion and wetting. Surface tension is a specific manifestation of surface energy in liquids, representing the force that minimizes the liquid surface area and influences droplet formation. Understanding the relationship between surface energy and surface tension is essential for optimizing adhesion in applications such as coatings, printing, and material bonding, where your control over these properties enhances performance.
Defining Surface Energy: Concepts and Importance
Surface energy refers to the excess energy at the surface of a material compared to its bulk, arising from unbalanced molecular forces. It plays a crucial role in adhesion, influencing how strongly your materials interact at interfaces. Understanding surface energy helps optimize coating, bonding, and wettability processes by promoting better adhesion through precise surface treatments.
Understanding Surface Tension in Liquids
Surface tension in liquids arises from cohesive forces between molecules at the liquid's surface, creating a measurable energy known as surface energy. This energy drives phenomena like droplet formation and adhesion, influencing how liquids interact with solid surfaces and determining wetting behavior. Understanding surface tension helps you control processes in coatings, adhesives, and fluid mechanics by optimizing surface energy to achieve desired adhesion properties.
Key Differences Between Surface Energy and Surface Tension
Surface energy quantifies the work needed to create a unit area of surface on a solid, reflecting the cohesive forces within the material, whereas surface tension represents the force per unit length acting along a liquid's surface, driven by molecular attractions. Surface energy influences adhesion by dictating the extent to which liquids can wet and bond to solids, while surface tension governs the shape and stability of liquid interfaces. Understanding these distinctions is crucial for applications in coating, adhesion, and material science, where controlling interfacial interactions affects performance and durability.
The Role of Adhesion in Surface Phenomena
Surface energy and surface tension both describe the interactions at the interface of materials, with adhesion playing a critical role in these surface phenomena. Adhesion governs how liquids spread or stick to solid surfaces by influencing the balance between cohesive forces within the liquid and adhesive forces between liquid and solid. Understanding this dynamic allows you to optimize applications such as coating, printing, and bonding by controlling wetting behavior and improving material compatibility.
Surface Energy vs. Surface Tension: Scientific Principles
Surface energy refers to the excess energy at the surface of a material compared to its bulk, stemming from unbalanced molecular forces, while surface tension is the force per unit length at the liquid-air interface, caused by cohesive molecular interactions. Surface energy primarily governs solid-liquid adhesion by influencing wettability and contact angle hysteresis, whereas surface tension dictates the shape and stability of liquid droplets and films. Understanding the interplay between surface energy and surface tension is crucial for applications in coatings, adhesion, and material science, as it determines interfacial phenomena and adhesion strength.
Measurement Techniques for Surface Energy and Surface Tension
Surface energy is typically measured using contact angle goniometry, where the angle formed by a liquid droplet on a solid surface provides insights into adhesion properties and the energy at the interface. Surface tension, often quantified through methods like the pendant drop or Wilhelmy plate techniques, reflects the cohesive forces at a liquid-air interface. Understanding these measurement techniques allows you to accurately characterize material interactions crucial for coatings, adhesion, and wetting processes.
Real-World Applications of Surface Energy and Adhesion
Surface energy and surface tension significantly influence adhesion in various industries, including automotive coatings, where optimal surface energy enhances paint adhesion and durability. In electronics manufacturing, controlling surface energy improves the bonding of components through better adhesive wetting and reduced defects. Medical device fabrication relies on tailored surface energy to ensure biocompatible adhesion of implants and coatings, enhancing performance and patient safety.
Influence of Material Properties on Surface Characteristics
Surface energy directly reflects a material's molecular cohesion, influencing surface tension, which governs adhesive interactions with liquids. Materials with high surface energy, such as metals and oxides, exhibit strong adhesive forces due to greater molecular attractions at the interface. Polymers and low-surface-energy materials display weaker surface tension, reducing adhesion and altering wettability on their surfaces.
Future Trends and Innovations in Surface Science
Advancements in nanotechnology and materials science are driving future trends in surface energy and surface tension research, enabling precise control over adhesion properties for diverse applications. Innovations such as smart coatings and programmable surfaces are enhancing the manipulation of surface energy at the molecular level, improving durability, self-cleaning, and bio-compatibility. You can expect these developments to revolutionize industries from healthcare to electronics by optimizing adhesion for specific functional requirements.
surface energy vs surface tension (adhesion) Infographic
 electrown.com
electrown.com