Sol-gel synthesis offers precise control over material composition and porosity at relatively low temperatures, making it ideal for producing homogeneous and highly pure nanoparticles. Hydrothermal synthesis excels in crystallizing complex structures under high pressure and temperature, yielding materials with enhanced crystallinity and unique morphologies; explore the rest of the article to understand which method suits your specific application needs.
Table of Comparison
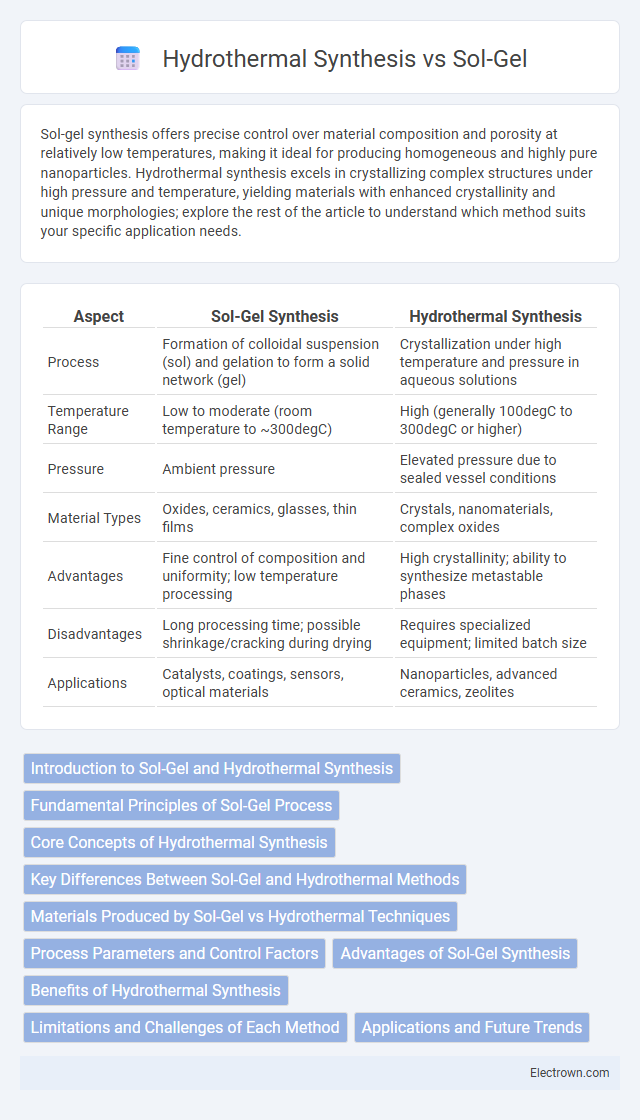
| Aspect | Sol-Gel Synthesis | Hydrothermal Synthesis |
|---|---|---|
| Process | Formation of colloidal suspension (sol) and gelation to form a solid network (gel) | Crystallization under high temperature and pressure in aqueous solutions |
| Temperature Range | Low to moderate (room temperature to ~300degC) | High (generally 100degC to 300degC or higher) |
| Pressure | Ambient pressure | Elevated pressure due to sealed vessel conditions |
| Material Types | Oxides, ceramics, glasses, thin films | Crystals, nanomaterials, complex oxides |
| Advantages | Fine control of composition and uniformity; low temperature processing | High crystallinity; ability to synthesize metastable phases |
| Disadvantages | Long processing time; possible shrinkage/cracking during drying | Requires specialized equipment; limited batch size |
| Applications | Catalysts, coatings, sensors, optical materials | Nanoparticles, advanced ceramics, zeolites |
Introduction to Sol-Gel and Hydrothermal Synthesis
Sol-gel synthesis is a versatile chemical process involving the transition of a system from a liquid "sol" into a solid "gel" phase, enabling the fabrication of metal oxides with controlled porosity and nanostructure. Hydrothermal synthesis employs aqueous solutions at elevated temperatures and pressures to crystallize materials, often producing highly crystalline metal oxides and other inorganic compounds with unique morphologies. Both methods offer distinct advantages in tailoring the physical and chemical properties of advanced materials for applications in catalysis, sensors, and energy storage.
Fundamental Principles of Sol-Gel Process
The sol-gel process involves the transition of a system from a liquid "sol" into a solid "gel" phase through hydrolysis and polycondensation of metal alkoxides or metal salts. This method enables precise control over the material's microstructure, homogeneity, and porosity at relatively low temperatures. Your choice of sol-gel synthesis allows for tailored nanoparticle formation and complex oxide materials essential in coatings, sensors, and catalysts.
Core Concepts of Hydrothermal Synthesis
Hydrothermal synthesis involves crystallizing substances from high-temperature aqueous solutions under high pressure, enabling the formation of highly crystalline materials with controlled morphology. This method typically operates at temperatures above 100degC and pressures exceeding atmospheric levels, facilitating the growth of nanomaterials and complex oxides that are difficult to produce via sol-gel processes. Your choice between hydrothermal and sol-gel synthesis depends on the desired material properties, as hydrothermal techniques excel in producing well-defined, dense crystals with fewer defects.
Key Differences Between Sol-Gel and Hydrothermal Methods
Sol-gel synthesis involves the transition of a system from a liquid "sol" into a solid "gel" phase through hydrolysis and polycondensation reactions, typically operating at low temperatures, making it suitable for producing uniform oxide materials with controlled porosity. Hydrothermal synthesis uses high-pressure and high-temperature aqueous solutions to promote crystal growth, enabling the formation of highly crystalline materials with unique morphologies that are often unattainable by conventional methods. Your choice between these techniques should consider factors such as operating conditions, material crystallinity requirements, and desired particle size or morphology.
Materials Produced by Sol-Gel vs Hydrothermal Techniques
Sol-gel synthesis primarily produces metal oxides and hybrid organic-inorganic materials with high purity and uniform particle size, often used in coatings, sensors, and catalysts. Hydrothermal synthesis yields crystalline materials such as zeolites, nanocrystals, and complex oxides with controlled morphology and superior crystallinity, ideal for advanced functional materials. The choice between sol-gel and hydrothermal methods depends on the desired material phase, particle size, and application-specific properties.
Process Parameters and Control Factors
Sol-gel synthesis involves precise control of parameters such as pH, temperature, and precursor concentration, enabling fine-tuning of particle size and porosity through hydrolysis and condensation reactions. Hydrothermal synthesis relies on high pressure and temperature conditions within sealed reactors, allowing control over crystallinity, morphology, and phase formation by adjusting factors like reaction time, temperature, and mineralizers. Both methods require meticulous parameter regulation to tailor nanomaterial properties for specific applications but differ in the processing environment and temperature-pressure regimes.
Advantages of Sol-Gel Synthesis
Sol-gel synthesis offers precise control over the chemical composition and homogeneity of materials at the nanoscale, enabling the creation of highly pure and uniform nanopowders or thin films. This method operates at relatively low temperatures, reducing energy consumption and allowing for the integration of temperature-sensitive components into your final product. The versatility of sol-gel techniques facilitates the fabrication of complex shapes and coatings with enhanced surface area, improving material performance in applications such as catalysis and sensors.
Benefits of Hydrothermal Synthesis
Hydrothermal synthesis offers precise control over crystal size, morphology, and phase purity by operating under high temperature and pressure conditions, which promotes enhanced material crystallinity. This method enables the formation of complex nanostructures and highly uniform particles that are difficult to achieve with sol-gel processes. Additionally, hydrothermal synthesis often results in superior material stability and improved functional properties for applications in catalysis, electronics, and energy storage.
Limitations and Challenges of Each Method
Sol-gel synthesis faces challenges such as long processing times, cracking during drying, and limited control over particle size distribution, which can affect the material's uniformity and properties. Hydrothermal synthesis often requires high pressure and temperature conditions, posing safety risks and demanding specialized equipment, while also offering limited scalability for industrial production. Choosing the right method depends on Your application's tolerance for these limitations and the desired material characteristics.
Applications and Future Trends
Sol-gel synthesis is extensively used in producing advanced ceramics, coatings, and optical materials due to its precise control over composition and nanostructure. Hydrothermal synthesis excels in fabricating crystalline materials like zeolites, metal oxides, and novel nanostructures with enhanced catalytic and electronic properties. Your choice between these methods will influence future innovations in energy storage, environmental remediation, and biomedicine, where scalability and material performance are critical factors.
sol-gel vs hydrothermal synthesis Infographic
 electrown.com
electrown.com