XRD (X-ray Diffraction) identifies crystal structures and phases by analyzing diffraction patterns, while XRF (X-ray Fluorescence) determines elemental composition through emitted secondary X-rays. Explore the article to understand which technique suits your material analysis needs best.
Table of Comparison
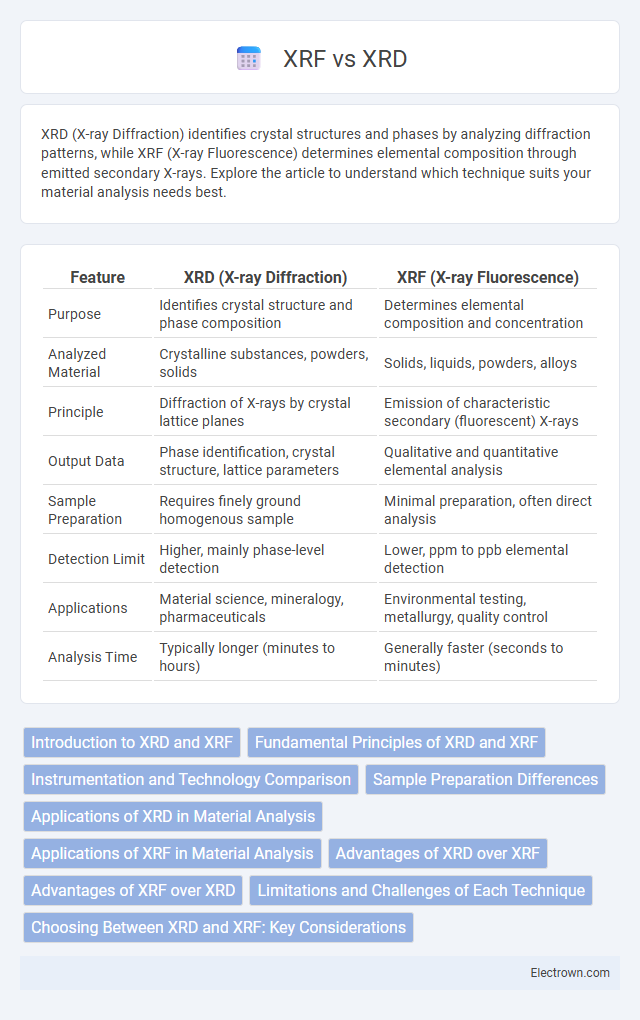
| Feature | XRD (X-ray Diffraction) | XRF (X-ray Fluorescence) |
|---|---|---|
| Purpose | Identifies crystal structure and phase composition | Determines elemental composition and concentration |
| Analyzed Material | Crystalline substances, powders, solids | Solids, liquids, powders, alloys |
| Principle | Diffraction of X-rays by crystal lattice planes | Emission of characteristic secondary (fluorescent) X-rays |
| Output Data | Phase identification, crystal structure, lattice parameters | Qualitative and quantitative elemental analysis |
| Sample Preparation | Requires finely ground homogenous sample | Minimal preparation, often direct analysis |
| Detection Limit | Higher, mainly phase-level detection | Lower, ppm to ppb elemental detection |
| Applications | Material science, mineralogy, pharmaceuticals | Environmental testing, metallurgy, quality control |
| Analysis Time | Typically longer (minutes to hours) | Generally faster (seconds to minutes) |
Introduction to XRD and XRF
X-ray diffraction (XRD) analyzes the crystallographic structure of materials by measuring the angles and intensities of diffracted X-rays, providing detailed information about phase identification and lattice parameters. X-ray fluorescence (XRF) determines the elemental composition by detecting characteristic secondary X-rays emitted from a sample when excited by a primary X-ray source, enabling rapid and non-destructive qualitative and quantitative analysis. Your choice between XRD and XRF depends on whether you require detailed structural information or elemental composition profiling.
Fundamental Principles of XRD and XRF
XRD (X-ray Diffraction) analyzes the atomic structure of crystalline materials by measuring the diffraction patterns produced when X-rays interact with the crystal lattice, revealing phase identification and crystallinity. XRF (X-ray Fluorescence) detects elemental composition by measuring characteristic secondary (fluorescent) X-rays emitted from a sample when excited by a primary X-ray source, enabling precise elemental quantification. Understanding these fundamental principles allows you to select the appropriate technique based on whether structural analysis (XRD) or elemental analysis (XRF) is required.
Instrumentation and Technology Comparison
XRD (X-ray Diffraction) utilizes a monochromatic X-ray source and detector system to analyze crystal structures by measuring the diffraction patterns of X-rays interacting with atomic planes. In contrast, XRF (X-ray Fluorescence) employs an X-ray tube or radioactive source that excites atoms in the sample, causing them to emit characteristic secondary X-rays detected for elemental composition analysis. Your choice between XRD and XRF instrumentation depends on whether structural crystallography or precise elemental quantification is the primary analytical goal.
Sample Preparation Differences
XRD sample preparation requires finely powdered, homogenous samples to ensure accurate diffraction patterns and minimal preferred orientation effects. In contrast, XRF sample preparation involves pressing powders into pellets or analyzing solids directly with minimal sample alteration. Proper sample prep for XRD often demands more time-consuming grinding and mounting steps, whereas XRF prioritizes surface flatness and uniform thickness for precise elemental analysis.
Applications of XRD in Material Analysis
X-ray Diffraction (XRD) is essential in material analysis for identifying crystalline phases and determining crystal structures, enabling precise characterization of metals, ceramics, and polymers. Unlike X-ray Fluorescence (XRF), which primarily provides elemental composition, XRD reveals detailed information on lattice parameters, crystallinity, and phase purity, crucial for quality control and research in materials science. Your ability to analyze microstructural properties, such as grain size and strain, using XRD greatly enhances material performance and development.
Applications of XRF in Material Analysis
X-ray fluorescence (XRF) is extensively used in material analysis for rapid, non-destructive elemental composition determination across metals, ceramics, polymers, and geological samples. XRF enables precise quantification of trace elements and alloy identification, crucial for quality control in manufacturing and mining exploration. Your ability to analyze coatings, contaminants, and compositional variations makes XRF invaluable for material characterization and verification.
Advantages of XRD over XRF
X-ray diffraction (XRD) offers superior capabilities in determining the crystallographic structure, phase identification, and quantitative phase analysis of materials compared to X-ray fluorescence (XRF), which primarily provides elemental composition. XRD can analyze crystalline materials to reveal detailed information about lattice parameters, crystallite size, and strain, enabling comprehensive mineralogical and materials science studies. The technique is highly effective for complex mixtures and distinguishing polymorphs, advantages where XRF lacks specificity.
Advantages of XRF over XRD
X-ray fluorescence (XRF) offers significant advantages over X-ray diffraction (XRD) in elemental analysis by providing rapid, non-destructive, and multi-element detection without the need for extensive sample preparation. XRF can analyze a wide range of materials, including solids, liquids, and powders, with higher sensitivity for trace elements, making it ideal for qualitative and quantitative elemental composition. The portability and ease of use of handheld XRF devices further enhance in-field analysis capabilities, unlike XRD, which typically requires larger, laboratory-based instruments.
Limitations and Challenges of Each Technique
XRD (X-ray Diffraction) faces limitations in analyzing amorphous materials and requires intricate sample preparation to obtain accurate crystalline phase identification, while complex peak overlap can hinder precise interpretation. XRF (X-ray Fluorescence) struggles with detecting light elements and is sensitive to matrix effects, which can reduce quantification accuracy without proper calibration. Your choice between XRD and XRF should consider these challenges in relation to the material composition and analysis goals.
Choosing Between XRD and XRF: Key Considerations
Selecting between X-ray Diffraction (XRD) and X-ray Fluorescence (XRF) depends on the specific analytical goals and material characteristics. XRD excels in identifying crystalline phases and determining crystal structure, making it essential for mineralogy and materials science. XRF provides rapid elemental composition analysis with minimal sample preparation, ideal for screening metal alloys and environmental samples.
XRD vs XRF Infographic
 electrown.com
electrown.com