Galvanic sensors operate by generating a current through the chemical reaction of oxygen at the electrode, providing quick and reliable oxygen measurements without an external power source. Polarographic sensors require an external voltage to reduce oxygen at the cathode, offering high sensitivity and accuracy but with greater complexity; discover which sensor suits Your application best by reading the full article.
Table of Comparison
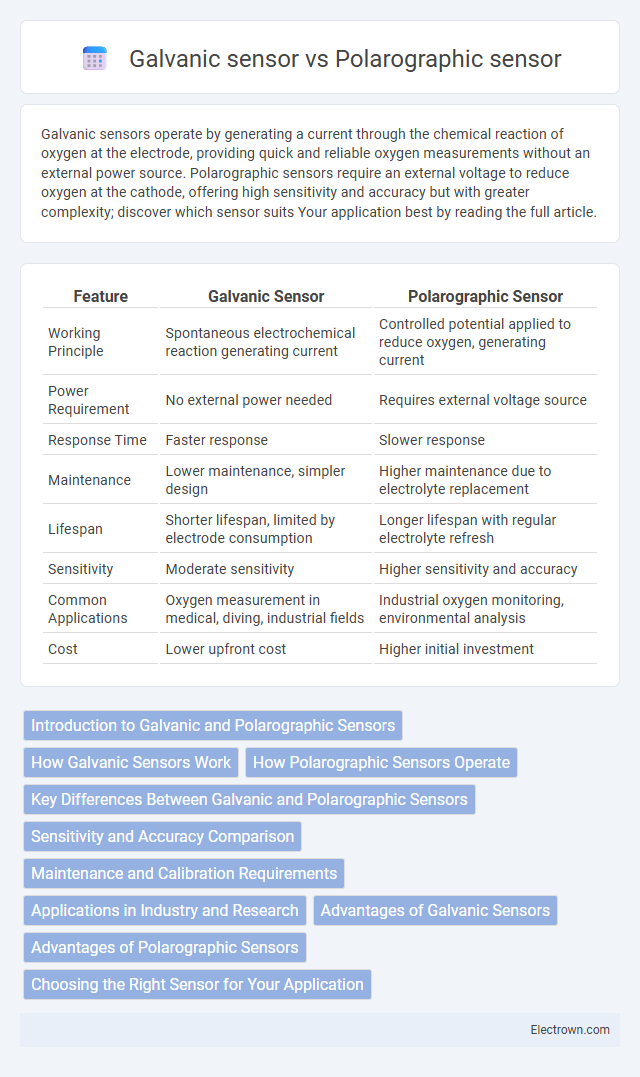
| Feature | Galvanic Sensor | Polarographic Sensor |
|---|---|---|
| Working Principle | Spontaneous electrochemical reaction generating current | Controlled potential applied to reduce oxygen, generating current |
| Power Requirement | No external power needed | Requires external voltage source |
| Response Time | Faster response | Slower response |
| Maintenance | Lower maintenance, simpler design | Higher maintenance due to electrolyte replacement |
| Lifespan | Shorter lifespan, limited by electrode consumption | Longer lifespan with regular electrolyte refresh |
| Sensitivity | Moderate sensitivity | Higher sensitivity and accuracy |
| Common Applications | Oxygen measurement in medical, diving, industrial fields | Industrial oxygen monitoring, environmental analysis |
| Cost | Lower upfront cost | Higher initial investment |
Introduction to Galvanic and Polarographic Sensors
Galvanic sensors operate based on a spontaneous redox reaction that generates an electrical current proportional to the gas concentration, commonly used for oxygen detection. Polarographic sensors utilize an applied voltage to drive a reduction reaction at the cathode, producing a measurable current related to analyte levels such as oxygen or other gases. Both sensor types provide sensitive and accurate electrochemical measurements but differ in operation principles and response characteristics.
How Galvanic Sensors Work
Galvanic sensors operate by generating a spontaneous electrochemical reaction between an anode and cathode immersed in an electrolyte, producing a measurable current proportional to the oxygen concentration. This current flow occurs without an external power source, differentiating galvanic sensors from polarographic sensors that require an applied voltage. The sensor's efficiency and response time depend on electrode materials, electrolyte composition, and membrane permeability, making it ideal for portable oxygen measurements in medical and environmental applications.
How Polarographic Sensors Operate
Polarographic sensors operate by measuring the current resulting from the reduction or oxidation of an electroactive species at a dropping mercury electrode under a controlled voltage. This electrochemical reaction generates a current proportional to the concentration of the target analyte, commonly oxygen. The sensor's performance relies on the precise control of applied potential and the diffusion rate of analytes to the electrode surface, enabling accurate and rapid detection in various industrial and environmental applications.
Key Differences Between Galvanic and Polarographic Sensors
Galvanic sensors generate a continuous current through spontaneous electrochemical oxidation without an external power source, while polarographic sensors require an applied voltage to measure the reduction or oxidation of analytes at the working electrode. Galvanic sensors are typically simpler, faster in response, and require less maintenance, whereas polarographic sensors offer greater sensitivity and selectivity due to controlled voltage sweeps. The primary difference lies in operation mode: galvanic sensors rely on self-generated voltage from chemical reactions, whereas polarographic sensors depend on externally applied potentials to produce measurable currents.
Sensitivity and Accuracy Comparison
Galvanic sensors typically offer faster response times and higher sensitivity at lower concentrations of gases compared to polarographic sensors, making them suitable for detecting trace levels with improved detection limits. Polarographic sensors provide more stable and accurate readings over extended periods due to their controlled electrolyte consumption and minimized signal drift. Your choice between the two should consider the specific accuracy requirements and sensitivity needs of your application environment.
Maintenance and Calibration Requirements
Galvanic sensors require minimal maintenance due to their simple design, often needing only periodic membrane replacement and electrolyte replenishment. Polarographic sensors demand more frequent calibration and maintenance to ensure accuracy, as their cathode can be susceptible to fouling and electrolyte depletion. Both sensors benefit from regular calibration, but polarographic models typically involve more complex procedures and shorter intervals between servicing.
Applications in Industry and Research
Galvanic sensors are widely used in industrial applications such as corrosion monitoring, environmental gas detection, and medical oxygen measurement due to their stability and low maintenance requirements. Polarographic sensors find extensive application in research settings for precise electrochemical analysis, including dissolved oxygen measurement in water quality studies and metabolic monitoring in biological systems. Both sensor types offer critical insights; galvanic sensors excel in durability for long-term industrial processes, while polarographic sensors provide high sensitivity and accuracy for detailed experimental data.
Advantages of Galvanic Sensors
Galvanic sensors offer a self-powered operation, eliminating the need for an external power source, which enhances portability and ease of use in various applications. Their faster response time compared to polarographic sensors allows for more immediate detection of gases like oxygen, making them highly effective in safety monitoring environments. The simpler design of galvanic sensors reduces maintenance requirements and increases durability, providing cost-effective and reliable performance in harsh or remote conditions.
Advantages of Polarographic Sensors
Polarographic sensors provide highly accurate and sensitive measurements of dissolved oxygen due to their stable polarization voltage and quick response time. They typically require less maintenance than galvanic sensors, as they have fewer moving parts and more consistent electrode surfaces. Your oxygen monitoring system benefits from improved durability and reliability when using polarographic sensors in demanding environments.
Choosing the Right Sensor for Your Application
Galvanic sensors provide rapid oxygen detection with minimal power consumption, ideal for portable and low-maintenance environments, while polarographic sensors offer higher accuracy and stability suited for laboratory and industrial applications requiring precise measurements. Consider factors like response time, calibration frequency, operating temperature range, and sensor lifespan when selecting between galvanic and polarographic sensors. Evaluating these parameters ensures optimal performance and reliability tailored to your specific oxygen monitoring needs.
Galvanic sensor vs Polarographic sensor Infographic
 electrown.com
electrown.com