Silicon (Si) and Germanium (Ge) are both group 14 elements widely used in semiconductor technology, with silicon being more abundant and cost-effective, while germanium offers superior electron mobility and faster operation speeds. Discover how these differences impact your choice in electronic applications by reading the rest of the article.
Table of Comparison
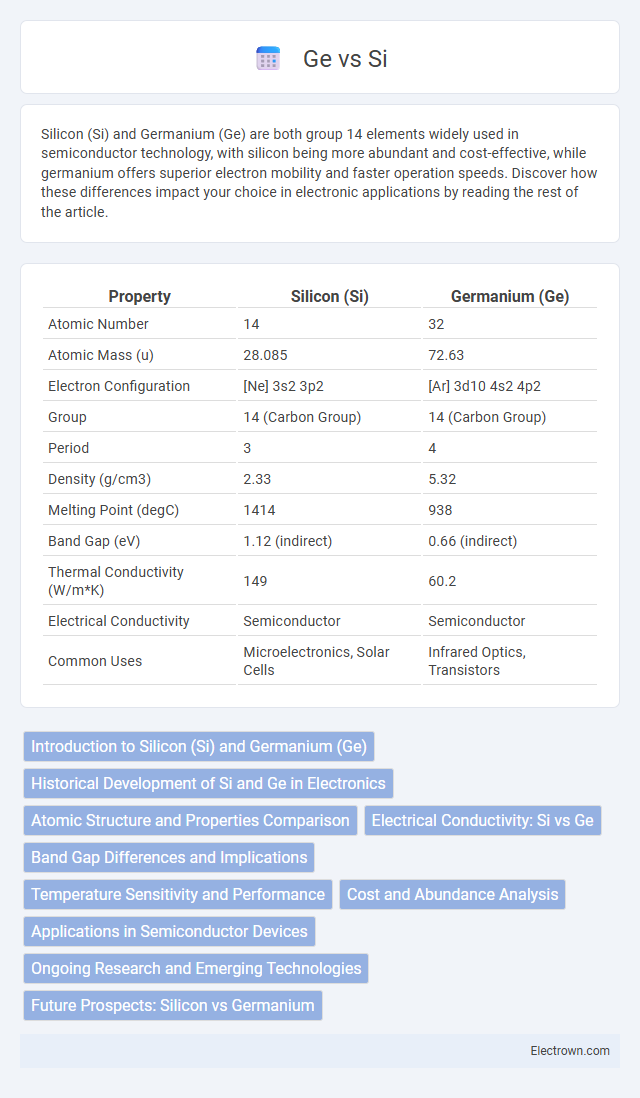
| Property | Silicon (Si) | Germanium (Ge) |
|---|---|---|
| Atomic Number | 14 | 32 |
| Atomic Mass (u) | 28.085 | 72.63 |
| Electron Configuration | [Ne] 3s2 3p2 | [Ar] 3d10 4s2 4p2 |
| Group | 14 (Carbon Group) | 14 (Carbon Group) |
| Period | 3 | 4 |
| Density (g/cm3) | 2.33 | 5.32 |
| Melting Point (degC) | 1414 | 938 |
| Band Gap (eV) | 1.12 (indirect) | 0.66 (indirect) |
| Thermal Conductivity (W/m*K) | 149 | 60.2 |
| Electrical Conductivity | Semiconductor | Semiconductor |
| Common Uses | Microelectronics, Solar Cells | Infrared Optics, Transistors |
Introduction to Silicon (Si) and Germanium (Ge)
Silicon (Si) and Germanium (Ge) are group IV semiconductors essential for modern electronics, with Si being the most widely used material in integrated circuits due to its abundant availability and superior thermal stability. Germanium offers higher electron mobility compared to silicon, making it advantageous for high-speed devices and photonics applications. Both elements form the backbone of semiconductor technology, but Si's native oxide layer (SiO2) provides better insulation properties, which has driven its dominance in the microelectronics industry.
Historical Development of Si and Ge in Electronics
Silicon (Si) and germanium (Ge) played pivotal roles in the evolution of semiconductor technology, with germanium dominating early transistor development in the 1940s due to its superior electron mobility. Silicon later surpassed germanium during the 1950s and 1960s because of its higher thermal stability and the formation of a robust silicon dioxide insulating layer, essential for integrated circuits. The transition from germanium to silicon catalyzed advancements in microelectronics, enabling the mass production of more reliable and efficient semiconductor devices.
Atomic Structure and Properties Comparison
Silicon (Si) and Germanium (Ge) have atomic numbers 14 and 32, respectively, with Si featuring 14 protons and electrons and Ge 32, resulting in different electron configurations that influence their chemical behaviors. Si has a smaller atomic radius (about 111 pm) compared to Ge (about 122 pm), which affects their bonding and electrical properties. Your understanding of these differences is crucial when choosing semiconductor materials, as Si offers higher thermal stability while Ge provides greater electron mobility.
Electrical Conductivity: Si vs Ge
Germanium (Ge) exhibits higher electrical conductivity than silicon (Si) due to its smaller band gap of 0.66 eV compared to silicon's 1.12 eV, which allows for easier electron excitation at room temperature. Intrinsic germanium's conductivity typically ranges between 2.0 to 4.0 x 10^-3 S/cm, while intrinsic silicon's conductivity is significantly lower at approximately 1.5 x 10^-6 S/cm. These electrical properties make Ge more suitable for high-speed electronic applications, whereas Si is favored for its thermal stability and abundance in semiconductor devices.
Band Gap Differences and Implications
Silicon (Si) has an indirect band gap of approximately 1.12 eV, while Germanium (Ge) features a smaller indirect band gap of about 0.66 eV, resulting in different electronic and optical properties. The narrower band gap in Ge enables higher carrier mobility and improved performance in high-speed electronics and infrared photodetectors. Consequently, Si is preferred for standard semiconductor applications due to its thermal stability and oxide quality, whereas Ge is favored in specialized devices requiring enhanced sensitivity and speed.
Temperature Sensitivity and Performance
Silicon (Si) exhibits lower temperature sensitivity compared to Germanium (Ge), making it more stable for electronic devices operating across a wide temperature range. Germanium offers higher electron mobility, enhancing performance at lower temperatures but suffers from increased leakage currents as temperature rises. Device performance in Si remains reliable at elevated temperatures, whereas Ge's efficiency degrades faster, limiting its use in high-temperature applications.
Cost and Abundance Analysis
Silicon (Si) is significantly more abundant in the Earth's crust, making it far less expensive compared to germanium (Ge), which is much rarer and sourced primarily as a byproduct of zinc refining. The high abundance of silicon translates to lower raw material and production costs, benefiting industries like semiconductor manufacturing and solar panel production. Your choice between Si and Ge will largely depend on budget constraints and availability, with silicon offering a cost-effective solution for large-scale applications.
Applications in Semiconductor Devices
Silicon (Si) dominates semiconductor devices due to its excellent thermal stability and native oxide layer, essential for integrated circuits and microprocessors. Germanium (Ge) offers superior electron mobility, making it valuable in high-speed and high-frequency applications such as photodetectors and infrared optics. You can leverage Si for cost-effective, reliable electronics while Germanium enhances performance in specialized devices requiring faster operation and sensitivity to infrared light.
Ongoing Research and Emerging Technologies
Ongoing research in silicon (Si) and germanium (Ge) focuses on enhancing electronic and photonic device performance by leveraging their complementary properties, such as Si's mature manufacturing ecosystem and Ge's higher charge carrier mobility. Emerging technologies explore Si-Ge heterostructures and nanostructured materials to improve transistor speed, energy efficiency, and integration with silicon photonics for faster data communication. Innovations in Si-Ge alloys and quantum dot applications continue to push the boundaries of semiconductor devices in computing, sensing, and optoelectronics.
Future Prospects: Silicon vs Germanium
Silicon remains the cornerstone of the semiconductor industry due to its abundant availability, mature fabrication processes, and excellent thermal stability, ensuring its dominance in future electronic devices. Germanium, with its higher electron and hole mobility, offers promising advantages for high-speed and optoelectronic applications, positioning it as a complementary material to silicon in next-generation technologies like 5G and photonics. Your choice between silicon and germanium will depend on the balance between cost-efficiency and performance requirements in emerging semiconductor innovations.
Si vs Ge Infographic
 electrown.com
electrown.com